Precision and Biocompatibility: Leveraging Metal 3D Printing for Biomedical Sensor Frames
Índice
The landscape of medical technology is constantly evolving, driven by the relentless pursuit of more accurate diagnostics, personalized treatments, and enhanced patient monitoring. At the heart of many groundbreaking medical devices lie sophisticated sensors, capable of detecting subtle physiological changes, transmitting vital data, and enabling timely interventions. However, the effectiveness of these sensors often hinges on the precision, stability, and biocompatibility of their surrounding structures – the frames that house, protect, and position them. Traditionally, manufacturing these intricate frames involved complex machining or molding processes, often limited by geometric constraints, material options, and production lead times. Today, metal additive manufacturing (AM), commonly known as metal Impresión 3D, is revolutionizing how these critical components are designed and produced, offering unprecedented advantages for engineers, designers, and medical device manufacturers. This post delves into the world of 3D printed biomedical sensor frames, exploring their applications, the compelling reasons to adopt metal AM, and the crucial role of material selection in ensuring safety and performance.
Introduction: The Critical Role of Frames in Biomedical Sensing Technology
Biomedical sensors are the unsung heroes of modern medicine. From monitoring glucose levels in diabetic patients to tracking heart rhythms via implantable devices or enabling minimally invasive surgical tools, these sensors provide critical real-time data that informs diagnoses and guides treatments. A biomedical sensor frame, in its essence, is the engineered structure that performs several vital functions:
- Housing and Protection: It encases the delicate sensing elements, shielding them from physical damage, bodily fluids, and environmental factors that could compromise their function or longevity.
- Positioning and Stability: The frame ensures the sensor is precisely located relative to the target tissue, organ, or fluid being monitored. This is crucial for accurate readings, especially in dynamic environments within the human body or during patient movement.
- Integration: It often facilitates the integration of the sensor with other components, such as power sources, data transmission units, or the larger medical device assembly. This includes providing secure mounting points, channels for wiring, or features for sealing.
- Biocompatibilidad: For implantable or body-contact applications, the frame material must be biocompatible, meaning it does not elicit harmful immune responses or toxic effects when interacting with biological tissues.
The demand for smaller, smarter, and more personalized medical devices is surging across various healthcare sectors:
- Wearable Technology: Fitness trackers have evolved into sophisticated health monitors capable of tracking ECG, blood oxygen saturation, and even continuous glucose levels. The frames for these sensors need to be lightweight, durable, skin-friendly, and capable of housing complex electronics in compact forms.
- Implantable Devices: Pacemakers, cochlear implants, neurostimulators, and implantable drug delivery systems rely on sensors integrated within highly reliable, biocompatible housings designed for long-term residence within the human body. Miniaturization and anatomical conformity are key challenges.
- Diagnostic Equipment: High-precision sensors are integral to laboratory equipment, imaging systems (like MRI or CT scanners), and point-of-care diagnostic tools. Their frames must guarantee stability, accuracy, and sometimes resistance to harsh cleaning or sterilization processes.
- Surgical Tools: Modern surgical instruments increasingly incorporate sensors for navigation, tissue characterization, or force feedback. The frames must be robust, sterilizable, and often intricately shaped to fit within minimally invasive tools.
Meeting the complex geometric, material, and performance requirements for these diverse applications using traditional manufacturing methods like CNC machining or injection molding can be challenging and costly. Machining intricate internal features or undercuts is difficult, tooling for molding is expensive and time-consuming (especially for low-to-mid volumes or custom designs), and material choices can be limited.
This is where metal additive manufacturing emerges as a powerful enabler. Impresión 3D en metal technologies, such as Selective Laser Melting (SLM) or Electron Beam Melting (EBM) – core technologies utilized by leading providers like Met3dp – build parts layer by layer directly from a 3D CAD model using high-performance metal powders. This approach fundamentally changes the design and production paradigm, offering solutions uniquely suited to the demands of biomedical sensor frames:
- Geometric Complexity: AM excels at creating complex internal channels, lattice structures for weight reduction, and organic shapes that conform to anatomy – features often impossible or prohibitively expensive to machine.
- Personalización: Patient-specific sensor frames or devices tailored to unique anatomical requirements become feasible without the need for custom tooling.
- Creación rápida de prototipos: Design iterations can be printed and tested quickly, dramatically accelerating the development cycle for new medical devices.
- Versatilidad de materiales: AM processes work with a range of biocompatible metals like Titanium alloys and Stainless Steels, essential for medical applications.
As a leader in metal AM, Met3dp provides not only industry-leading printing equipment known for its accuracy and reliability but also specializes in the production of high-performance metal powders optimized for these demanding applications. Understanding the critical role of sensor frames and the transformative potential of metal AM sets the stage for exploring where this technology is making the most significant impact.

Applications: Where are 3D Printed Sensor Frames Making an Impact?
The versatility of metal additive manufacturing allows it to address the specific needs of various biomedical sensor applications, driving innovation across the healthcare spectrum. Procurement managers and engineers in medical device Original Equipment Manufacturers (OEMs), contract manufacturing organizations (CMOs), and research labs are increasingly turning to 3D printed metal frames to overcome traditional manufacturing limitations and unlock new device capabilities.
1. Wearable Health Monitors: The trend towards continuous, remote patient monitoring fuels the growth of sophisticated wearable devices. These devices require sensor frames that are:
- Lightweight and Comfortable: For long-term wear without user burden. AM enables the creation of complex lattice structures within the frame, significantly reducing weight while maintaining structural integrity.
- Durable and Robust: To withstand daily wear and tear. Metals like 316L stainless steel offer excellent durability and corrosion resistance.
- Compact and Integrated: Housing sensors, batteries, and microelectronics in a small footprint. AM allows for part consolidation, integrating mounting features and enclosures directly into the frame design.
- Skin-Contact Safe: Utilizing biocompatible materials like medical-grade 316L or Titanium alloys where necessary.
- Example: Frames for wearable ECG monitors requiring precise electrode positioning, or housings for continuous glucose monitoring sensors that need to be robust yet discreet. B2B suppliers focusing on wearable medical device components find AM ideal for producing these custom, complex parts efficiently.
2. Implantable Sensors and Devices: This is arguably where metal AM offers the most profound advantages due to the stringent requirements for biocompatibility, long-term reliability, and anatomical fit.
- Biocompatibilidad: Materials like Ti-6Al-4V ELI (Extra Low Interstitials) are widely used due to their proven safety profile within the human body. Metal AM processes, when properly controlled, can produce dense, non-corrosive parts from these materials. Met3dp’s focus on high-purity, contamination-free powders is critical here.
- Customization and Anatomical Fit: AM allows the creation of patient-specific implant frames based on medical imaging data (CT/MRI scans). This improves device fit, reduces surgery time, and potentially enhances therapeutic outcomes. Think of custom pacemaker enclosures or cranial implants with integrated sensor mounts.
- Miniaturization and Complexity: Housing increasingly complex sensors (e.g., for neural interfaces, intraocular pressure monitoring) in minimal space. AM can create intricate internal structures and features necessary for these advanced implants.
- Osteointegración: For implants interacting with bone, AM can create porous surface structures or lattices designed to encourage bone ingrowth, improving long-term stability.
- Example: Housings for neurostimulator leads incorporating sensor feedback loops, frames for implantable drug delivery systems requiring precise positioning, or structural components for cochlear implants. Companies seeking custom implant manufacturing o implantable sensor housing solutions are prime candidates for metal AM services.
3. Diagnostic Equipment Components: While not implanted, sensors within diagnostic and laboratory equipment require extreme precision and stability.
- High Precision and Stability: Frames must hold sensors rigidly and accurately, often within complex assemblies. Metal AM can achieve tight tolerances and produce dimensionally stable parts. Met3dp printers, for instance, are noted for their industry-leading accuracy.
- Geometrías complejas: Integrating cooling channels, mounting points for optics or electronics, or flow paths for fluidic sensors. AM handles this complexity with ease compared to multi-part machined assemblies.
- Compatibilidad de materiales: Frames might need resistance to cleaning agents or specific chemicals used in assays. 316L stainless steel is often a suitable choice.
- Example: Mounting brackets for sensors within an MRI machine requiring non-magnetic properties (specific Titanium grades), frames holding optical sensors in blood analysis equipment, or components for microfluidic diagnostic chips requiring precise channel dimensions. Procurement teams looking for a reliable diagnostic equipment parts supplier can benefit from AM’s flexibility.
4. Surgical Tool Sensors: Modern surgery leverages sensor technology for enhanced feedback and navigation.
- Robustness and Sterilizability: Frames must withstand the rigors of surgery and repeated sterilization cycles (autoclaving). Both Ti-6Al-4V ELI and 316L are suitable and can be readily sterilized.
- Miniaturization: Integrating sensors into the tips of minimally invasive surgical tools or catheters. AM excels at creating these small, intricate components.
- Integration: Designing frames that seamlessly integrate sensors, wiring, and the tool’s mechanical structure.
- Example: Frames housing force sensors in robotic surgical arms, brackets for navigation sensors on orthopedic surgical guides, or housings for pressure sensors in catheter tips. Medical device companies developing advanced surgical systems rely on manufacturing partners capable of producing these custom medical sensors and components.
Across these diverse applications, metal 3D printing provides engineers and manufacturers with a powerful toolset. It enables the creation of biomedical sensor frames that are not only functional and reliable but also optimized for specific performance requirements, patient needs, and integration challenges, paving the way for the next generation of medical devices. Identifying reliable medical device contract manufacturing partners with proven AM expertise is crucial for OEMs aiming to leverage this technology effectively.
Why Choose Metal 3D Printing for Sensor Frames? Unlocking Key Advantages
While traditional manufacturing methods like CNC machining and metal injection molding (MIM) have served the medical device industry well, metal additive manufacturing presents a compelling value proposition specifically for biomedical sensor frames, driven by advantages in design freedom, speed, customization, and supply chain efficiency. For engineers designing intricate medical components and procurement managers seeking cost-effective, high-quality advanced medical manufacturing solutions, understanding these benefits is key.
1. Unmatched Design Freedom & Complexity:
- Límites tradicionales: CNC machining struggles with internal cavities, undercuts, and highly complex organic shapes, often requiring parts to be made in multiple pieces and then assembled. MIM requires expensive tooling, making complex designs costly and inflexible to change.
- Ventaja AM: AM builds parts layer by layer, allowing for the creation of virtually any geometry imaginable directly from a CAD file. This enables:
- Canales internos: For cooling, wiring, or fluid flow within the sensor frame.
- Estructuras reticulares: To significantly reduce weight while maintaining strength, crucial for wearables and implants.
- Consolidación de piezas: Combining multiple components of a sensor assembly into a single printed part, reducing assembly time, potential failure points, and overall cost.
- Organic & Conformal Shapes: Designing frames that perfectly match patient anatomy (for implants) or ergonomic requirements (for wearables).
- Impacto: Engineers are no longer constrained by manufacturability limitations, enabling more innovative and optimized sensor frame designs.
2. Rapid Prototyping and Accelerated Development:
- Límites tradicionales: Creating prototypes via machining can be time-consuming, and creating molds for MIM is slow and expensive, making design iteration a lengthy process.
- Ventaja AM: Metal AM allows for the direct printing of functional prototypes from CAD data, often within days. This facilitates:
- Quick Design Validation: Engineers can quickly test form, fit, and function with real materials.
- Faster Iteration Cycles: Multiple design variations can be printed and evaluated in parallel or sequence, leading to optimized designs much faster.
- Reducción del plazo de comercialización: Accelerating the prototyping phase significantly shortens the overall medical device development timeline.
- Impacto: Medical device companies can innovate faster, respond more quickly to clinical feedback, and bring potentially life-saving devices to market sooner. This is a crucial benefit for firms involved in medical device prototyping.
3. Customization and Patient-Specific Solutions:
- Límites tradicionales: Mass customization or creating one-off patient-specific devices is often economically unviable with traditional methods due to the high cost of custom tooling or complex machining setups.
- Ventaja AM: Since AM is tool-less, printing unique parts is essentially as easy as printing standard ones. This enables:
- Patient-Specific Implants: Sensor frames integrated into implants that perfectly match an individual’s anatomy derived from CT/MRI scans.
- Customized Wearables: Devices tailored to specific user groups or individual ergonomic needs.
- Low-Volume Production Runs: Economically producing niche devices or components in small batches.
- Impacto: AM enables a shift towards personalized medicine, where devices are tailored to the individual, potentially leading to better outcomes and patient satisfaction. This capability is transforming custom implant manufacturing.
4. Material Efficiency and Reduced Waste:
- Límites tradicionales: Subtractive manufacturing (machining) starts with a solid block of material and removes excess, often generating significant waste (up to 80-90% for complex parts), especially with expensive materials like medical-grade titanium.
- Ventaja AM: Additive manufacturing uses only the material needed to build the part and its supports. While some support material is used and some powder remains unfused, the overall material utilization is typically much higher than subtractive methods. Unfused powder can often be recycled and reused in subsequent builds (with proper quality control).
- Impacto: Reduced material consumption leads to cost savings, particularly with high-cost biocompatible alloys, and contributes to more sustainable manufacturing practices. This is a key consideration for cost-conscious procurement and environmentally aware organizations.
5. On-Demand Production and Supply Chain Flexibility:
- Límites tradicionales: Reliance on tooling and long machining setup times can lead to long lead times and the need for large inventory stockpiles.
- Ventaja AM: Digital inventory (CAD files) can be printed as needed, enabling:
- Inventario reducido: Minimizing the need for physical stock and warehousing costs.
- Distributed Manufacturing: Parts can potentially be printed closer to the point of need.
- Faster Response to Demand Fluctuations: Production can be scaled up or down more easily without tooling constraints.
- Impacto: AM offers greater agility and resilience in the medical device supply chain, enabling on-demand parts production and reducing the risks associated with inventory obsolescence or supply disruptions.
Comparison Table: AM vs. Traditional Methods for Sensor Frames
| Característica | Fabricación aditiva de metales (AM) | Mecanizado CNC | Moldeo por inyección de metal (MIM) |
|---|---|---|---|
| Complejidad del diseño | Very High (internal features, lattices, organic) | Moderado (limitado por el acceso a las herramientas) | High (but requires complex tooling) |
| Personalización | High (Ideal for patient-specific) | Low-to-Moderate (costly setup) | Very Low (requires new molds) |
| Velocidad de creación de prototipos | Very Fast (Days) | Moderate (Days to Weeks) | Slow (Weeks to Months for tooling) |
| Coste de utillaje | Ninguno | Low (fixtures) | Very High (molds) |
| Residuos materiales | Low-to-Moderate (powder recycling possible) | High (subtractive) | Low (near-net shape) |
| Volumen ideal | Low-to-Mid, Custom, Prototypes | Low-to-High (simpler parts) | High Volume |
| Lead Time (Prod.) | Moderado | Moderate-to-High | High (initial), Low (repeat) |
| Consolidación de piezas | Excelente | Limitado | Moderado |
Exportar a hojas
By carefully considering these advantages, engineers and procurement specialists can strategically leverage metal 3D printing services, such as those offered by Met3dp with their advanced equipment and material expertise, to produce superior biomedical sensor frames that meet the demanding requirements of modern healthcare.

Material Focus: Selecting Biocompatible Metals for Sensor Frame Integrity
The choice of material for a biomedical sensor frame is paramount. It dictates not only the mechanical performance and longevity of the device but also, critically, its interaction with the human body. For frames intended for implantation or prolonged contact with tissues and fluids, biocompatibility is non-negotiable. Metal additive manufacturing offers compatibility with several key medical-grade alloys, with Titanium Ti-6Al-4V ELI and Stainless Steel 316L being among the most prominent choices. Sourcing high-quality, certified powders from a reliable biocompatible metal powders supplier is the first step towards ensuring device safety and efficacy.
1. Titanium Alloy: Ti-6Al-4V ELI (Grade 23) Ti-6Al-4V ELI (Extra Low Interstitials) is often considered the workhorse material for medical implants, and for good reason. It’s an alpha-beta titanium alloy specifically processed to have reduced levels of oxygen, nitrogen, carbon, and iron compared to standard Ti-6Al-4V (Grade 5). This reduction in interstitial elements significantly improves its ductility and fracture toughness, particularly at cryogenic temperatures, though its primary benefit in medical applications is enhanced biocompatibility and fatigue strength.
- Propiedades clave:
- Excelente biocompatibilidad: Forms a stable, passive oxide layer (TiO2) upon exposure to air or bodily fluids, which prevents ion leakage and adverse tissue reactions. Widely accepted for long-term implantation (ISO 5832-3 standard).
- Alta relación resistencia-peso: Offers strength comparable to many steels but at nearly half the density, crucial for reducing the weight of implants and wearables.
- Excelente resistencia a la corrosión: Highly resistant to corrosion by bodily fluids, cleaning agents, and sterilization processes.
- Good Fatigue Strength: Important for devices subjected to cyclic loading within the body (e.g., components near joints or the heart).
- Non-Ferromagnetic: Safe for use in MRI environments.
- Osseointegration Potential: Can bond directly with bone tissue, especially when surface-treated or designed with porous structures achievable via AM.
- Typical Sensor Frame Applications:
- Implantable sensor housings (pacemakers, neurostimulators, cochlear implants).
- Frames integrated into orthopedic or dental implants.
- Components for long-term wearable monitors requiring maximum biocompatibility.
- Frames for implantable biosensors (e.g., glucose, pressure).
- AM Considerations: Ti-6Al-4V ELI is well-suited for both Laser Powder Bed Fusion (LPBF/SLM) and Electron Beam Melting (EBM) processes. EBM often results in lower residual stress but typically rougher surface finish compared to LPBF. Post-processing, such as stress relief heat treatment and potentially Hot Isostatic Pressing (HIP), is usually required to optimize mechanical properties and reduce internal porosity. Sourcing powder with controlled chemistry and particle size distribution is critical for consistent printing results. Met3dp’s expertise in producing specialized titanium alloys, including TiNi, TiTa, TiAl, and TiNbZr, alongside standard grades, highlights their capability in handling these advanced materials.
2. Stainless Steel: 316L 316L is an austenitic chromium-nickel stainless steel alloy with added molybdenum, enhancing its corrosion resistance, particularly against chlorides (like those found in bodily fluids). The “L” designates low carbon content (typically <0.03%), which minimizes carbide precipitation during welding or heat treatments, thus preserving corrosion resistance, especially at grain boundaries.
- Propiedades clave:
- Good Biocompatibility: While generally considered less biocompatible than Ti-6Al-4V ELI for permanent implants due to potential nickel ion release in sensitive individuals over long periods, 316L (meeting standards like ISO 5832-1) is widely accepted for temporary implants, surgical instruments, and external or short-term contact devices.
- Excelente resistencia a la corrosión: Highly resistant to general corrosion, pitting, and crevice corrosion in physiological environments.
- Buena resistencia y ductilidad: Offers a good balance of mechanical properties.
- Rentable: Generally less expensive than titanium alloys.
- Ease of Processing: Readily printable via LPBF and relatively easy to post-process (polish, machine).
- Sterilizable: Compatible with common sterilization methods like autoclaving.
- Typical Sensor Frame Applications:
- Frames for external wearable sensors (where skin contact is managed or brief).
- Components within diagnostic equipment not in direct long-term patient contact.
- Housings for sensors in surgical instruments.
- Temporary or short-term implantable device components (e.g., fixation devices with integrated sensing).
- Prototyping where final material might be titanium but cost is a factor initially.
- AM Considerations: 316L is one of the most common materials printed using LPBF. It generally prints well, achieving high density. Post-processing typically involves stress relief and surface finishing (e.g., polishing, electropolishing) to improve corrosion resistance and aesthetics. Ensuring the powder meets medical grade stainless steel specifications is crucial.
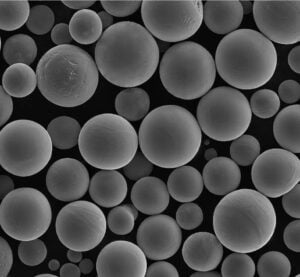
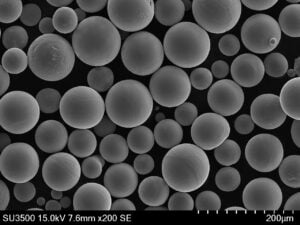
The Importance of Powder Quality and Sourcing Regardless of the chosen alloy, the quality of the metal powder used in the AM process is critical, especially for medical applications. Factors like:
- Pureza y química: Must meet stringent medical standards (e.g., ASTM F136 for Ti-6Al-4V ELI, ASTM F138 for 316L). Contaminants can compromise biocompatibility and mechanical properties.
- Distribución del tamaño de las partículas (PSD): Affects powder flowability, packing density, and the resolution achievable during printing.
- Esfericidad: Spherical particles flow better and pack more densely, leading to more uniform layers and denser final parts.
- Ausencia de satélites: Small particles clinging to larger ones can negatively impact flowability and spreadability.
- Low Porosity: Internal gas porosity within powder particles can translate to porosity in the final part.
Companies like Met3dp, employing advanced powder manufacturing techniques such as Atomización de gas de fusión por inducción al vacío (VIGA) y Proceso de electrodos rotativos de plasma (PREP), focus on producing high-quality spherical metal powders with precisely controlled characteristics. Their unique nozzle and gas flow designs in gas atomization, for example, aim for high sphericity and good flowability, which are essential for printing dense, high-quality metal powder for medical devices. Partnering with a supplier that understands and controls these powder characteristics, and provides material certifications and traceability, is fundamental for any medical device manufacturer utilizing metal AM.
Material Selection Table Summary
| Característica | Ti-6Al-4V ELI (Grado 23) | Acero inoxidable 316L |
|---|---|---|
| Primary Advantage | Superior Biocompatibility, Strength/Weight | Excellent Corrosion Resistance, Cost |
| Biocompatibilidad | Excellent (Long-term implant) | Good (Short-term/External, Instruments) |
| Densidad | ~4,43 g/cm³ | ~8,0 g/cm³ |
| Fuerza | Alta | Moderate-to-High |
| Resistencia a la corrosión | Excelente | Excellent (esp. vs. chlorides) |
| MRI Compatibility | Yes (Non-Ferromagnetic) | No (Generally Paramagnetic/Weakly Ferro) |
| Coste | Alta | Moderado |
| Common AM Process | LPBF, EBM | LPBF |
| Key Standard | ISO 5832-3, ASTM F136 | ISO 5832-1, ASTM F138 |
| Uso típico | Permanent Implants, High-Performance Wearables | Surgical Tools, Diagnostics, Ext. Devices |
Exportar a hojas
Selecting the right material involves balancing the technical requirements of the sensor frame (mechanical loads, environment), the necessary level of biocompatibility based on application (implant vs. external), regulatory pathways, and budget constraints. Consulting with materials experts and experienced AM service providers like Met3dp is crucial to making the optimal choice for your specific biomedical sensor frame application.
Design for Additive Manufacturing (DfAM): Optimizing Sensor Frames for 3D Printing
Simply taking a design intended for machining and sending it to a metal 3D printer rarely yields optimal results. To truly harness the power of AM, engineers must embrace Design for Additive Manufacturing (DfAM) principles. DfAM isn’t just about ensuring a part poder be printed; it’s about designing it intelligently to maximize AM’s advantages like complexity, consolidation, and lightweighting, while minimizing potential issues like excessive support structures, residual stress, or difficult post-processing. For intricate components like biomedical sensor frames, DfAM is critical.
Key DfAM Principles for Sensor Frames:
- Leveraging Geometric Complexity: AM’s greatest strength is its ability to create complex shapes. For sensor frames, this translates to:
- Internal Channels & Cavities: Design integrated channels for wiring, fiber optics, or even microfluidic pathways directly within the frame structure. This protects delicate components and reduces assembly complexity. Consider smooth, curved channels rather than sharp angles to facilitate powder removal during post-processing.
- Conformal Design: Shape frames to follow anatomical contours (for implants) or ergonomic forms (for wearables). This enhances fit, comfort, and potentially, sensor performance by ensuring optimal contact or positioning. Utilize patient-specific imaging data (CT/MRI) to create truly personalized frames.
- Feature Integration: Incorporate mounting points, snap-fits, alignment features, or connectors directly into the printed frame, eliminating the need for separate components and fasteners.
- Lightweighting with Lattice Structures and Topology Optimization:
- Estructuras reticulares: Replace solid volumes within the frame with internal lattice or gyroid structures. These highly complex, porous geometries, easily produced with AM, drastically reduce weight and material usage while maintaining required structural stiffness and strength. This is vital for implants (reducing stress shielding) and wearables (improving comfort). Different lattice cell types (e.g., cubic, octet-truss) offer varying mechanical properties.
- Optimización de la topología: Use software tools to algorithmically determine the most efficient material distribution within a defined design space, based on applied loads and constraints. This often results in organic-looking, highly optimized structures that remove material from low-stress areas, achieving maximum performance with minimum weight – ideal for high-performance sensor frames in demanding applications.
- Consolidación de piezas:
- Analyze existing sensor assemblies designed for traditional manufacturing. Identify opportunities to combine multiple individual components (brackets, housings, fasteners) into a single, monolithic printed part.
- Ventajas: Reduces part count, simplifies assembly, lowers inventory needs, eliminates potential failure points at joints or interfaces, and often reduces overall weight and production cost.
- Example: A sensor housing previously made of a machined body, a separate lid, and multiple screws might be redesigned as a single printed unit with integrated snap-fits or a bayonet closure mechanism.
- Designing for Minimal Support Structures:
- Support structures are often necessary in metal AM (especially LPBF) to anchor the part to the build plate, support overhanging features, and conduct heat away to prevent warping. However, supports consume extra material, add printing time, and require removal in post-processing, which can be challenging and potentially damage fine features or surfaces.
- Strategies:
- Orientación: Carefully select the part’s orientation on the build plate to minimize the extent of overhangs requiring support. Analyze trade-offs between support minimization, surface quality on critical faces, and print time.
- Ángulos autoportantes: Design overhangs to be below the critical angle (typically around 45 degrees from the horizontal for many metal AM processes, but process/material dependent) so they can be printed without supports. Use chamfers or fillets instead of sharp horizontal overhangs where possible.
- Internal Supports: Minimize or avoid complex internal supports, as they can be extremely difficult or impossible to remove, especially in small channels. Design internal features to be self-supporting or provide access ports for support removal and powder evacuation.
- Considering Wall Thickness and Fine Features:
- Metal AM processes have limitations on the minimum wall thickness and feature size they can reliably produce (influenced by laser/beam spot size, powder particle size, layer thickness).
- Guidelines: Typically, minimum wall thicknesses might be around 0.3-0.5 mm, but this varies significantly. Consult with your AM service provider, like Met3dp, regarding the specific capabilities of their métodos de impresión and materials. Avoid designing features close to the process limits unless absolutely necessary and validated.
- Ensure sufficient wall thickness for structural integrity, especially after potential surface finishing operations that remove material.
- Designing for Post-Processing:
- Anticipate downstream steps during the design phase.
- Support Removal Access: Ensure areas where supports attach are physically accessible for manual or CNC removal without damaging the part.
- Powder Evacuation: For parts with internal channels or cavities, design in strategically placed escape holes (which can potentially be plugged or welded shut later if needed) to facilitate complete removal of unfused powder. Trapped powder is unacceptable in medical devices.
- Tolerancias de mecanizado: If tight tolerances or specific surface finishes are required on certain features, design extra ‘stock’ material on those surfaces to be removed via CNC machining after printing.
- Acabado superficial: Consider which surfaces require high polish (e.g., for biocompatibility, friction reduction) and design them to be accessible for polishing tools.
La colaboración es clave: Effectively implementing additive manufacturing design guidelines requires collaboration between design engineers and AM process experts. Engaging with experienced service providers like Met3dp early in the design phase can provide invaluable feedback on manufacturability, material selection, orientation strategies, and potential cost implications, ultimately leading to a more robust and optimized biomedical sensor frame design. Their expertise across various productos and applications ensures practical, effective DfAM implementation.

Achieving Precision: Tolerance, Surface Finish, and Dimensional Accuracy Standards
For biomedical sensor frames, precision is often paramount. The frame must accurately position the sensor element, potentially seal against fluids or tissues, and fit correctly within a larger assembly or anatomical site. Understanding the levels of tolerance, surface finish, and overall dimensional accuracy achievable with metal AM is crucial for setting realistic expectations and ensuring the final part meets functional requirements.
1. Tolerances: Tolerance refers to the permissible limit or limits of variation in a physical dimension. Metal AM processes, while capable of high complexity, are not inherently as precise as high-end CNC machining in their as-built state.
- Typical As-Built Tolerances:
- Laser Powder Bed Fusion (LPBF/SLM): Generally offers higher precision. Typical tolerances might range from ±0.1 mm to ±0.2 mm for smaller dimensions (e.g., up to 50-100 mm), potentially increasing slightly for larger parts (e.g., ±0.1% to ±0.2% of the dimension). Specific feature tolerances can sometimes be tighter.
- Fusión por haz de electrones (EBM): Often has slightly looser tolerances than LPBF, perhaps in the range of ±0.2 mm to ±0.4 mm or ±0.4% of the dimension, due to the higher energy input and different powder characteristics.
- Factores que influyen en la tolerancia:
- Calibración de la máquina: Regular and precise calibration of the AM system is essential.
- Propiedades del material: Thermal expansion and shrinkage vary between materials (e.g., Ti-6Al-4V vs. 316L).
- Geometría y tamaño de la pieza: Large or complex parts with varying cross-sections can experience more thermal stress and distortion.
- Orientation & Supports: How the part is oriented and supported significantly impacts accuracy and potential warping.
- Gestión térmica: Build plate heating, process parameters (laser power, scan speed), and gas flow influence the thermal history and resulting stresses/accuracy.
- Post-procesamiento: Stress relief heat treatment can cause minor dimensional changes; machining introduces its own tolerances.
- Achieving Tighter Tolerances: When as-built tolerances are insufficient for critical features (e.g., mating surfaces, sealing interfaces, precise sensor locators), secondary machining is employed. Parts are printed with extra material (machining allowance) on critical surfaces, and then CNC machined to achieve tolerances as tight as ±0.01 mm to ±0.05 mm or better, comparable to traditional manufacturing. It’s vital to specify these requirements clearly on engineering drawings.
2. Surface Finish: Surface finish, often quantified by average roughness (Ra), describes the texture of a part’s surface. This is critical for biomedical applications due to its impact on biocompatibility, friction, sealing, fatigue life, and cleanability.
- As-Built Surface Finish:
- Metal AM parts typically have a rougher surface finish compared to machined parts. The roughness depends on the process, material, layer thickness, particle size, and surface orientation relative to the build direction.
- LPBF: Ra values often range from 6 µm to 15 µm (or higher) depending on the angle of the surface (upward-facing surfaces are generally smoother than downward-facing or side walls).
- EBM: Tends to produce rougher surfaces, potentially Ra 20 µm to 35 µm or more.
- Efecto Escalera: Curved or angled surfaces exhibit a characteristic “stair-step” texture due to the layered nature of the process.
- Mejora del acabado superficial: For most medical applications, especially implants or fluid-contact surfaces, the as-built finish is insufficient. Various post-processing techniques are used:
- Media Blasting (e.g., Bead Blasting): Provides a uniform matte finish, removes semi-sintered particles, but only slightly improves Ra (e.g., down to Ra 3-6 µm).
- Tumbling/Vibratory Finishing: Uses abrasive media in a rotating or vibrating drum to smooth surfaces and deburr edges. Can achieve Ra ~1-3 µm.
- Manual or CNC Polishing: Mechanical polishing using progressively finer abrasives can achieve very smooth, mirror-like finishes (Ra < 0.1 µm), often required for implant surfaces to maximize biocompatibility and reduce bacterial adhesion.
- Electropulido: An electrochemical process that removes a microscopic layer of material, preferentially from peaks, resulting in a very smooth, clean, and often more corrosion-resistant surface (common for 316L). Can achieve Ra ~0.2-0.8 µm.
- Specifying Requirements: Clearly define the required surface finish (e.g., specifying Ra max) on engineering drawings for critical surfaces. Understand that achieving very fine finishes adds cost and lead time.
3. Dimensional Accuracy: Dimensional accuracy refers to how closely the final part conforms to the nominal dimensions specified in the CAD model. It encompasses both tolerance (variation) and systematic deviations.
- Ensuring Accuracy:
- Control de procesos: Reliable AM service providers like Met3dp emphasize rigorous process control, machine calibration, and optimized parameter sets developed through experience to minimize deviations. Their printers are often highlighted for industry-leading print volume, accuracy and reliability.
- Simulation: Thermal simulation tools can sometimes predict potential distortion, allowing for design or support strategy adjustments preemptively.
- Quality Control & Inspection: Post-print measurement using CMM (Coordinate Measuring Machines), 3D scanning, or traditional metrology tools is essential to verify critical dimensions against specifications. CT scanning can verify internal features and dimensions.
- Iterative Refinement: For highly critical parts, initial prints might be used to characterize process deviations, allowing for compensation adjustments in the CAD model for subsequent prints to improve accuracy.
Summary Table: Precision Metrics in Metal AM
| Métrica | Typical As-Built Range (LPBF) | Typical As-Built Range (EBM) | Achievable with Post-Processing | Key Considerations for Sensor Frames |
|---|---|---|---|---|
| Tolerancia | ±0.1 to ±0.2 mm (±0.1-0.2%) | ±0.2 to ±0.4 mm (±0.4%) | ±0.01 to ±0.05 mm (via machining) | Sensor positioning, mating surfaces, sealing interfaces |
| Surface Finish (Ra) | 6 µm to 15+ µm | 20 µm to 35+ µm | < 0.1 µm (Polishing), ~0.4 µm (EP) | Biocompatibility, friction, sealing, cleanability, fatigue |
| Min. Feature Size | ~0.3 to 0.5 mm | Slightly larger than LPBF | Limited by AM process primarily | Miniaturization, thin walls, connector details |
Exportar a hojas
Engineers and procurement managers must understand these achievable precision levels. Specify critical tolerances and surface finishes clearly, and discuss expectations with the chosen AM provider to ensure the final dimensional accuracy AM parts meet the demanding requirements of biomedical sensor applications.
Beyond Printing: Essential Post-Processing Steps for Biomedical Sensor Frames
Creating a biomedical sensor frame doesn’t end when the 3D printer stops. The “as-built” part, while geometrically complete, requires several crucial post-processing steps to achieve the desired material properties, surface characteristics, cleanliness, and ultimately, fitness for use in a medical device. These steps are not optional extras; they are integral to ensuring the safety, performance, and biocompatibility of the final component. Understanding these post-processing metal AM parts requirements is vital for project planning, costing, and supplier evaluation.
Common Post-Processing Stages:
- Tratamiento térmico antiestrés:
- Why: The rapid heating and cooling cycles inherent in powder bed fusion processes (especially LPBF) induce significant residual stresses within the printed part. These stresses can cause distortion during or after removal from the build plate, reduce fatigue life, and potentially lead to premature failure.
- Proceso: Parts (often while still attached to the build plate) are heated in a controlled atmosphere (vacuum or inert gas like Argon) to a specific temperature below the alloy’s transformation point, held for a period, and then slowly cooled. This allows the material’s microstructure to relax, relieving internal stresses without significantly altering the core mechanical properties.
- Importancia: Absolutely essential for dimensional stability and mechanical integrity, particularly for materials like Ti-6Al-4V. Specific protocols (heat treatment Ti-6Al-4V) depend on the alloy and application requirements.
- Extracción de la pieza de la placa de montaje:
- Proceso: Parts are typically separated from the metal build plate using wire EDM (Electrical Discharge Machining) or a band saw.
- Consideraciones: Requires careful handling to avoid damaging the part. The separation surface will likely require subsequent finishing.
- Retirada de la estructura de soporte:
- Why: Supports are necessary during printing but must be removed afterwards.
- Proceso: Can range from simple manual breaking (for well-designed, accessible supports) to intricate manual work with hand tools, or CNC machining for more robust or difficult-to-access supports.
- Desafíos: Can be labor-intensive and time-consuming, especially for complex geometries with internal supports. Risk of damaging the part surface or fine features during removal. DfAM plays a huge role in simplifying this step. The surfaces where supports were attached often have lower quality and require additional finishing.
- Prensado isostático en caliente (HIP):
- Why: While AM processes aim for full density, microscopic internal pores (due to trapped gas or incomplete fusion) can sometimes remain. These pores can act as stress concentrators, significantly reducing fatigue life and fracture toughness – critical concerns for long-term implants.
- Proceso: Parts are subjected to high temperature (below melting point) and high isostatic pressure (typically using an inert gas like Argon) simultaneously in a specialized vessel. This combination collapses internal voids, effectively achieving full theoretical density (typically >99.9%).
- Importancia: Often mandatory for critical, fatigue-sensitive applications like orthopedic or cardiovascular implants made from Ti-6Al-4V ELI. It significantly improves mechanical properties, especially fatigue strength and ductility. Adds cost and lead time.
- Acabado superficial:
- Why: To achieve the required smoothness, cleanliness, biocompatibility, and aesthetic appearance, as the as-built surface is generally too rough for most medical applications.
- Procesos: As detailed previously – media blasting, tumbling, manual/CNC polishing, electropolishing. The chosen method(s) depend on the material, geometry, and specific surface requirements (Ra value, visual appearance). Medical device surface finishing often requires multiple steps to achieve the desired outcome.
- Cleaning and Passivation:
- Why: Extremely critical for medical devices to remove all contaminants from the manufacturing process. This includes unfused powder particles (especially from internal channels), residues from machining or polishing (oils, abrasives), fingerprints, and any other foreign material.
- Cleaning Process: Typically involves multiple stages, potentially including ultrasonic cleaning in specific detergents, solvent rinsing, and thorough drying. Validated cleaning procedures are essential. For parts with complex internal geometries, ensuring complete powder removal can be challenging and may require specific flow-through cleaning methods or verification via CT scanning.
- Passivation (esp. for Stainless Steels): A chemical process (often using nitric or citric acid) that removes free iron from the surface of stainless steel and enhances the natural chromium-rich passive oxide layer. This significantly improves corrosion resistance and biocompatibility by reducing the potential for ion leaching.
- Final Inspection and Quality Control:
- Why: To verify that the finished sensor frame meets all dimensional, surface finish, cleanliness, and material property specifications.
- Métodos: Dimensional inspection (CMM, 3D scanning), surface roughness measurement, visual inspection (including microscopic examination), NDT (Non-Destructive Testing) like CT scanning for internal integrity/powder removal, material certification review, cleanliness testing (e.g., bioburden tests).
Workflow Integration: These post-processing steps are interconnected and must be planned as part of the overall manufacturing workflow. For instance, machining might be done after stress relief but before final polishing and cleaning. The specific sequence depends on the part, material, and requirements. Partnering with an AM service provider like Met3dp, which offers comprehensive solutions including post-processing and quality assurance tailored for industries like medical, ensures these critical steps are managed effectively.

Navigating Challenges: Common Issues in 3D Printing Sensor Frames and Solutions
While metal AM offers tremendous advantages, it’s not without its challenges, particularly when producing complex, high-precision components like biomedical sensor frames. Awareness of potential issues and proactive implementation of mitigation strategies are key to successful outcomes. Reliable quality control additive manufacturing protocols are essential throughout the process.
1. Warping and Distortion:
- Asunto: Uneven heating and cooling during printing cause internal stresses that can lead to parts warping or distorting, especially thin or large flat structures.
- Causes: High thermal gradients, insufficient support, improper build plate heating, suboptimal scan strategy.
- Solutions:
- DfAM: Design modifications to reduce large flat areas or add stiffening features.
- Orientación: Choose an orientation that minimizes stress accumulation.
- Estrategia de apoyo: Use robust supports designed to anchor the part effectively and manage heat dissipation.
- Parámetros del proceso: Optimize laser/beam power, scan speed, and hatching patterns. Use build plate heating.
- Alivio del estrés: Perform stress relief heat treatment immediately after printing, often before removing the part from the build plate.
2. Support Structure Removal Difficulties:
- Asunto: Supports in hard-to-reach areas (internal channels, delicate features) can be difficult or impossible to remove completely without damaging the part. Residual support material is unacceptable.
- Causes: Complex part geometry, suboptimal orientation, overly dense or poorly designed supports.
- Solutions:
- DfAM: Design for minimal support (self-supporting angles, feature orientation). Design access points for internal support removal. Use topology optimization which often results in designs needing less support.
- Support Design: Utilize specialized support structures (e.g., thinner connection points, perforated supports) that are easier to remove.
- Orientación: Prioritize orientations that minimize internal supports or place them in accessible locations.
- Removal Techniques: Employ careful manual removal, micro-machining, or potentially electrochemical methods where applicable. Budget sufficient time and resources for this step.
3. Achieving Fine Feature Resolution and Thin Walls:
- Asunto: Difficulty in consistently and accurately producing very small features (e.g., pins, small holes) or thin walls below the process capability limits. Features may be incomplete, distorted, or lack definition.
- Causes: Laser/beam spot size limitations, powder particle size, heat accumulation in small features, layer thickness settings.
- Solutions:
- DfAM: Avoid designing features below the established process limits. Use fillets instead of sharp corners on thin sections.
- Machine/Process Selection: Choose a machine and parameter set optimized for high resolution if fine features are critical. EBM generally has lower resolution than LPBF.
- Parameter Optimization: Fine-tune laser power, scan speed, and focus specifically for small features (often requires expert knowledge).
- Material Choice: Some materials may resolve features better than others.
4. Incomplete Powder Removal:
- Asunto: Residual unfused powder trapped within internal channels, cavities, or complex lattice structures after printing and standard cleaning. This is a major biocompatibility risk for medical devices.
- Causes: Complex internal geometries with no escape paths, insufficient cleaning procedures, powder particle adhesion/sintering.
- Solutions:
- DfAM: Design internal channels with smooth curves, sufficient diameter, and dedicated entry/exit ports for powder removal and cleaning fluid circulation. Avoid dead-end cavities.
- Orientación: Orient the part to facilitate powder drainage during the build and breakout process.
- Procedimientos de limpieza: Employ rigorous, validated cleaning protocols potentially involving vibration, compressed air/gas flow, ultrasonic cleaning in specific solvents, and multiple rinse cycles.
- Inspection: Use methods like endoscopy or micro-CT scanning to verify complete powder removal from critical internal areas.
5. Surface Quality and Roughness:
- Asunto: As-built surface roughness, stair-stepping on curved surfaces, or partially sintered particles adhering to the surface may be unacceptable for function or biocompatibility.
- Causes: Layered process nature, powder particle size, melt pool dynamics, support contact points, down-facing surfaces.
- Solutions:
- Orientación: Optimize part orientation to place critical surfaces in upward-facing or vertical positions where possible.
- Parameter Optimization: Finer layer thickness and optimized contour scan parameters can improve sidewall quality.
- Post-procesamiento: Implement appropriate surface finishing techniques (blasting, tumbling, polishing, electropolishing) as required to meet specifications.
- DfAM: Use fillets instead of sharp edges where high finish is needed to mitigate stair-stepping effects.
6. Ensuring Biocompatibility and Cleanliness:
- Asunto: Ensuring the final part is free from contaminants (powder, processing fluids, bacterial endotoxins) and that the material itself doesn’t leach harmful ions or cause adverse reactions.
- Causes: Inadequate cleaning, improper material handling, cross-contamination, suboptimal material choice or processing.
- Solutions:
- Certificación de materiales: Use only certified medical-grade powders from reputable suppliers like Met3dp. Maintain strict material traceability.
- Control de procesos: Implement rigorous controls throughout the AM and post-processing workflow to prevent contamination. Use dedicated equipment for medical parts if possible.
- Validated Cleaning: Develop and validate cleaning procedures specifically for the part geometry and material to ensure removal of all residues down to acceptable levels (often requiring chemical analysis or biological testing).
- Biocompatibility Testing: Perform necessary biocompatibility tests (e.g., cytotoxicity, sensitization, implantation tests per ISO 10993) on final, sterilized parts or representative coupons produced via the exact same process chain.
Successfully troubleshooting 3D printing defects and challenges requires a combination of DfAM expertise, meticulous process control, appropriate post-processing, and rigorous quality assurance. Partnering with an experienced AM provider who understands the specific demands of the medical industry is crucial for navigating these complexities and delivering safe, effective biomedical sensor frames.
Partner Selection: How to Choose the Right Metal 3D Printing Service Provider
Not all metal AM service providers are created equal, especially when it comes to the stringent requirements of the medical device industry. Selecting a partner involves evaluating far more than just their printing capabilities; it requires a thorough assessment of their quality systems, material expertise, regulatory compliance, technical support, and overall experience with medical applications. Finding a capable and trustworthy medical device contract manufacturer specializing in AM is key.
Critical Evaluation Criteria:
- Quality Management System (QMS) and Certifications:
- ISO 13485 Certification: This is the international standard for QMS for medical device manufacturing. While not all AM providers hold this certification (as they might supply components to certified device manufacturers), a provider with ISO 13485 demonstrates a commitment to the rigorous process controls, documentation, traceability, and risk management required for medical components. It significantly streamlines the qualification process for medical device OEMs. Even if not certified themselves, inquire deeply into their QMS structure and how it aligns with ISO 13485 principles.
- Certificación ISO 9001: A foundational certification for quality management, indicating established processes for consistency and customer satisfaction. It’s a minimum requirement for any serious industrial manufacturing partner.
- Robust QMS Elements: Look for evidence of well-documented procedures for order entry, process validation, operator training, equipment calibration and maintenance, material handling and traceability, non-conformance management, corrective and preventive actions (CAPA), and final inspection. Quality assurance additive manufacturing should be deeply embedded in their operations.
- Material Expertise and Traceability:
- Medical-Grade Material Handling: Do they have experience printing with the specific biocompatible alloys you need (e.g., Ti-6Al-4V ELI per ASTM F136, 316L per ASTM F138)?
- Powder Sourcing and Control: Where do they source their powders? Do they use certified medical-grade powders? What are their procedures for incoming powder inspection, handling, storage (to prevent contamination and degradation), and recycling/reuse (including tracking powder batches and limiting reuse cycles)? Reputable providers like Met3dp, which manufacture their own high-quality spherical metal powders using advanced VIGA and PREP technologies, offer a distinct advantage in controlling powder quality from the source.
- Material Certifications and Traceability: Can they provide full material certifications (certificates of conformity/analysis) for each powder batch used? Do they maintain traceability linking the final part back to the specific powder batch, machine run, and processing parameters used? This is non-negotiable for medical devices.
- Equipment Capabilities and Process Control:
- Technology Match: Do they operate the appropriate AM technology (e.g., LPBF, EBM) best suited for your sensor frame’s material, complexity, and required features?
- Machine Quality and Maintenance: Are they using industrial-grade AM systems known for reliability and accuracy? What are their calibration and preventative maintenance schedules? Met3dp emphasizes its industry-leading print volume, accuracy and reliability for mission-critical parts.
- Process Monitoring and Validation: Do they employ in-situ monitoring capabilities (e.g., melt pool monitoring)? Have they validated their printing processes for the specific materials and parameters used for medical components? Ask for evidence of process stability and repeatability.
- Post-Processing Capabilities and Validation:
- In-House vs. Outsourced: Do they perform critical post-processing steps (stress relief, HIP, machining, polishing, cleaning) in-house or through qualified subcontractors? In-house capabilities often provide better control over the entire workflow and potentially shorter lead times.
- Expertise and Equipment: Do they have the necessary equipment and expertise for required steps like high-precision CNC machining, various surface finishing techniques, and validated cleaning processes suitable for medical devices?
- Validation: Have their post-processing steps, especially cleaning and potentially sterilization compatibility, been validated?
- Engineering Support and DfAM Expertise:
- Colaboración: Are they willing to work collaboratively with your engineering team early in the design phase?
- DfAM Guidance: Can they provide expert advice on optimizing the sensor frame design for additive manufacturing (DfAM), including feedback on orientation, support strategies, feature feasibility, and cost reduction opportunities?
- Resolución de problemas: Do they have experienced application engineers who can help troubleshoot potential manufacturing challenges? Met3dp, with its decades of collective expertise, explicitly offers application development services alongside its printers and powders.
- Experience with Medical Devices:
- Track Record: Can they demonstrate successful experience in producing components for medical applications, particularly parts with similar complexity or material requirements? Ask for (non-confidential) case studies or examples.
- Understanding of Regulatory Landscape: Do they understand the regulatory context (e.g., FDA requirements, MDR in Europe) and the importance of documentation and validation for medical device components?
- Inspection and Metrology Capabilities:
- Equipamiento: Do they possess adequate inspection equipment (CMM, 3D scanners, surface profilometers, visual inspection tools, potentially NDT like CT scanning) to verify that parts meet all specifications?
- Informar: Can they provide detailed inspection reports documenting compliance with critical dimensions, tolerances, and surface finish requirements?
- Confidentiality and IP Protection:
- NDAs: Are they willing to sign Non-Disclosure Agreements (NDAs) to protect your sensitive intellectual property?
- Security: What measures do they have in place to ensure data security for your CAD files and project information?
- Communication, Project Management, and Logistics:
- Capacidad de respuesta: Are they responsive to inquiries and provide clear communication throughout the project lifecycle?
- Gestión de proyectos: Do they assign a dedicated point of contact? How do they manage project timelines and provide updates?
- Location and Shipping: Consider their location relative to yours for shipping times and costs, although for high-value medical components, capabilities often outweigh proximity.
Auditoría de proveedores: For critical medical components, conducting an on-site or thorough remote audit of potential suppliers against these criteria is highly recommended before establishing a partnership. Choosing the right partner is an investment in quality, reliability, and ultimately, patient safety. Companies like Met3dp, positioning themselves as providers of comprehensive solutions spanning printers, advanced powders, and application services, aim to meet these demanding criteria for industries like aerospace, medical, and automotive.

Understanding Investment: Cost Factors and Typical Lead Times for Production
While metal AM offers significant advantages, it’s essential to understand the factors driving the cost and lead time for producing biomedical sensor frames. This allows for accurate budgeting, realistic project planning, and informed decisions when comparing AM to traditional methods or evaluating quotes from different service providers. Requesting detailed quotes for metal 3D printing cost estimation is standard practice.
Principales factores de coste:
- Coste del material:
- Alloy Type: Biocompatible metals like Ti-6Al-4V ELI are significantly more expensive per kilogram than medical-grade 316L stainless steel or other non-medical alloys.
- Powder Consumption: This depends on the part volume (including any machining stock) and the volume of support structures required. Efficient DfAM (e.g., lightweighting, minimizing supports) directly reduces material consumption.
- Calidad del polvo: High-purity, tightly controlled powders required for medical applications typically command a premium price.
- Machine Time / Print Time:
- Part Volume & Height: Larger parts or taller parts (in the build orientation) take longer to print.
- Number of Layers: Determined by part height and selected layer thickness (finer layers increase print time but improve surface finish/resolution).
- Estrategia de escaneo: The complexity of the laser/beam scanning path affects time.
- Máquina Tarifa por hora: Service providers factor in the amortization, maintenance, operation, and overhead costs of their expensive industrial AM systems. Rates vary based on machine type, size, and location.
- Nesting Efficiency: Printing multiple parts simultaneously in one build (nesting) can significantly reduce the per-part machine time cost by sharing setup and cycle times. This is crucial for servicios de impresión 3D al por mayor aiming for volume production.
- Costes laborales:
- Setup: Preparing the build file, setting up the machine, loading powder.
- Breakout: Removing the build plate, separating parts, initial powder removal/cleaning.
- Retirada del soporte: Often a manual or semi-automated process requiring skilled technicians; complexity heavily influences this cost.
- Post-procesamiento: Labor for machining, polishing, cleaning, inspection, etc. Highly skilled labor for tasks like intricate polishing can be a significant cost factor.
- Engineering/Programming: DfAM consultation, build preparation optimization, or CNC programming for post-machining might incur separate fees or be built into the overall cost.
- Estructuras de apoyo:
- Material Volume: Supports consume material, adding to cost.
- Removal Cost: As noted above, the labor and time required for removal is a direct cost. Difficult-to-remove supports significantly increase this.
- Costes de postprocesamiento:
- Tratamiento térmico: Furnace time, energy consumption, inert gas usage.
- HIP: A specialized, high-cost process typically outsourced, adding significantly to per-part cost but essential for critical applications.
- Mecanizado: CNC machine time, tooling, programming, and labor costs. Cost depends heavily on the extent of machining required and tolerance demands.
- Acabado superficial: Costs vary greatly depending on the method (blasting is relatively cheap, multi-stage manual polishing is expensive) and the required final Ra value.
- Cleaning & Passivation: Costs associated with specialized equipment, chemicals, validated processes, and labor.
- Quality Assurance and Inspection:
- Nivel de inspección: Basic dimensional checks vs. comprehensive CMM reports, surface roughness measurements, NDT (CT scanning), cleanliness testing – each adds cost.
- Documentación: Generating detailed traceability and quality documentation required for medical devices takes time and resources.
- Cantidad del pedido:
- Setup Amortization: Fixed setup costs are amortized over the number of parts in a batch. Higher quantities generally lead to lower per-part costs.
- Descuentos por volumen: Providers may offer tiered pricing for larger production runs.
Typical Lead Times:
Additive manufacturing lead times are influenced by several factors, and it’s crucial to understand the breakdown:
- Quoting & Order Processing: Can range from hours (for automated online quotes on simple parts) to several days (for complex parts requiring manual review and DfAM consultation).
- Queue Time: Depending on the service provider’s machine availability and backlog, your job may wait in a queue before printing starts. This can range from days to weeks.
- Print Time: Highly variable based on part size, complexity, and nesting. Can range from several hours for small parts to multiple days for large parts or full build plates.
- Post-Processing Time: This can often take significantly longer than the print time itself. Stress relief, HIP (if needed, often requires batching and scheduling), support removal, machining, extensive polishing, and rigorous cleaning/inspection cycles can add days or even weeks to the total lead time.
- Envío: Standard shipping times based on location.
Estimated Timelines (General Guidance):
- Prototypes (Simple, minimal post-processing): 5 – 15 business days
- Prototypes (Complex, extensive post-processing): 2 – 4 weeks
- Low-Volume Production (e.g., < 50 units): 3 – 6 weeks
- Mid-Volume Production (e.g., 100s of units): 5 – 10 weeks+ (highly dependent on part and process complexity)
Lo más importante: Obtain detailed quotes specifying all included steps and associated costs. Discuss lead time expectations early and understand the time required for each stage, particularly post-processing, to build realistic project schedules.
Frequently Asked Questions (FAQ) about 3D Printed Biomedical Sensor Frames
Here are answers to some common questions engineers and procurement managers have when considering metal AM for biomedical sensor frames:
Q1: Are 3D printed metal sensor frames truly biocompatible? A: Yes, provided the correct procedures are followed. Biocompatibility depends primarily on:
- Material Choice: Using certified medical-grade alloys known for biocompatibility (like Ti-6Al-4V ELI conforming to ASTM F136 or implant-grade 316L per ASTM F138) is the foundation.
- Control de procesos: Ensuring the AM process itself doesn’t introduce contaminants and achieves high density to prevent unwanted leaching. Partnering with providers experienced in medical applications, like Met3dp, who emphasize powder purity and process control is vital.
- Post-procesamiento: Thorough, validated cleaning is absolutely critical to remove all residual powder particles and processing fluids. Surface treatments like polishing or electropolishing can further enhance biocompatibility by creating smoother, more passive surfaces.
- Pruebas: Final confirmation often requires biocompatibility testing (per ISO 10993 standards) on parts produced using the exact manufacturing process (printing + all post-processing steps), especially for implantable devices.
Q2: What level of detail, miniaturization, and thin walls can realistically be achieved for sensor frames? A: The achievable level depends on the specific AM process (LPBF generally offers higher resolution than EBM), the machine used, the material, and optimized process parameters.
- Tamaño mínimo de característica: Typically around 0.2 mm to 0.4 mm, but this can vary. Very small positive features (like pins) can be challenging due to heat concentration. Small holes might need secondary drilling/reaming for high precision.
- Espesor mínimo de pared: Generally around 0.3 mm to 0.5 mm, but thicker walls are recommended for structural integrity, especially if significant post-processing (like polishing) is required. Designing below these limits requires careful validation.
- Overall Miniaturization: AM excels at creating complex geometries in small packages, enabling significant miniaturization compared to assembling multiple machined parts. Discuss specific feature requirements with your AM provider early in the design phase.
Q3: How does the cost of metal 3D printing compare to traditional manufacturing (CNC Machining, MIM) for sensor frames? A: The cost comparison depends heavily on part complexity y production volume:
- Complejidad: For highly complex geometries (internal channels, lattices, organic shapes) that are difficult or impossible to machine, AM is often more cost-effective even at low volumes because it avoids complex setups, tooling, and assembly steps.
- Volumen:
- Prototypes & Low Volume (1-100s): AM is typically very competitive or cheaper than CNC (due to no tooling and minimal setup for complexity) and much cheaper than MIM (which has very high tooling costs).
- Mid Volume (100s-1000s): This is often the “cross-over” zone. Highly complex parts might still favor AM, while simpler parts may become cheaper via CNC. MIM starts to become viable if complexity isn’t extreme.
- High Volume (10,000s+): For simpler geometries, CNC machining or especially MIM (if the part is suitable) will generally be more cost-effective than current metal AM processes due to faster cycle times and lower per-part costs at scale.
- Other Factors: AM’s ability to consolidate parts can lead to system-level cost savings (reduced assembly, inventory). Lead time advantages of AM for prototyping can also represent significant value.
Q4: Are 3D printed Ti-6Al-4V ELI and 316L sensor frames compatible with standard medical sterilization methods? A: Yes. Both Ti-6Al-4V ELI and 316L stainless steel, whether wrought or produced via AM with proper post-processing (especially cleaning), are compatible with common medical sterilization methods, including:
- Autoclaving (Steam Sterilization): The most common method.
- Ethylene Oxide (EtO) Gas Sterilization: Suitable, but requires proper aeration afterwards.
- Gamma Radiation Sterilization: Generally compatible, although very high doses could potentially affect some material properties minimally (less concern for metals than polymers).
- Low-Temperature Hydrogen Peroxide Gas Plasma: Also generally compatible. It’s crucial that the parts are thoroughly cleaned before sterilization, as any residual contaminants could compromise the process or lead to device failure.
Q5: How can we verify that complex internal channels within a sensor frame are free of residual powder? A: This is a critical concern, especially for implantable devices. Verification methods include:
- Design for Inspection (DfI): Where possible, design channels with entry/exit ports large enough for visual inspection using borescopes or micro-cameras.
- Process Validation: Rigorously validate the cleaning process using test coupons or representative parts, potentially analyzing rinse fluids for particle counts.
- Ensayos no destructivos (END): Alta resolución Micro-Computed Tomography (Micro-CT scanning) is the most effective method for non-destructively visualizing and confirming the absence of powder within complex internal geometries. While it adds cost, it provides definitive evidence and is often employed for critical medical components during process validation or on a sampling basis during production.
Addressing these FAQ metal 3D printing medical questions highlights the importance of technical understanding and close collaboration with knowledgeable AM partners.
Conclusion: The Future is Precise – Additive Manufacturing for Advanced Biomedical Sensors
The intricate world of biomedical sensors demands manufacturing solutions that offer precision, material integrity, design freedom, and the ability to create increasingly complex and personalized components. As we’ve explored throughout this post, metal additive manufacturing rises to this challenge, providing a powerful toolkit for engineers and medical device manufacturers striving for innovation.
From enabling lightweight, patient-specific implant frames in Ti-6Al-4V ELI to producing robust, integrated sensor housings in 316L for diagnostic tools, the advantages of impresión 3D en metal are clear:
- Libertad de diseño sin precedentes: Creating complex internal channels, integrated features, and organic shapes previously unattainable.
- La personalización en masa: Enabling patient-specific solutions and economically viable low-volume production.
- Accelerated Innovation: Rapid prototyping drastically shortens development cycles.
- Optimized Performance: Lightweighting through lattices and topology optimization enhances device function and patient comfort.
- Materiales de alto rendimiento: Compatibility with proven biocompatible alloys like Ti-6Al-4V ELI and 316L.
However, harnessing this potential requires more than just access to a printer. It demands a holistic approach encompassing strategic Design for Additive Manufacturing (DfAM), meticulous attention to process control, rigorous and validated post-processing (especially cleaning), and stringent quality assurance. Navigating the potential challenges – from managing residual stress to ensuring complete powder removal – necessitates expertise and diligence.
The selection of a manufacturing partner becomes a critical success factor. Look for providers who demonstrate not only technical proficiency but also a deep understanding of the medical industry’s unique requirements for quality, traceability, and compliance. Partners like Met3dp, who offer comprehensive solutions – from developing and manufacturing advanced, high-purity spherical metal powders and industry-leading printing systems to providing expert application development services – are invaluable allies in this journey. Their commitment to quality and innovation aligns perfectly with the needs of the future of medical manufacturing.
Metal additive manufacturing is no longer a futuristic novelty; it is a present-day reality that is actively shaping the next generation of biomedical sensors and devices. By embracing AM, companies can create more effective diagnostic tools, less invasive surgical instruments, more comfortable wearables, and truly personalized implants, ultimately contributing to better patient outcomes. As the technology continues to mature, offering greater precision, faster speeds, and an expanding portfolio of materials, its impact on healthcare innovation will only continue to grow.
To explore how Met3dp’s cutting-edge systems, high-performance metal powders, and deep expertise in additive manufacturing can empower your organization’s development of advanced biomedical sensor frames and other critical medical components, visit Met3dp or contact their team today. Let’s build the future of precision healthcare, together.
Compartir
MET3DP Technology Co., LTD es un proveedor líder de soluciones de fabricación aditiva con sede en Qingdao, China. Nuestra empresa está especializada en equipos de impresión 3D y polvos metálicos de alto rendimiento para aplicaciones industriales.
Solicite información para obtener el mejor precio y una solución personalizada para su empresa.
Artículos relacionados

Segmentos de álabe de tobera de alto rendimiento: Revolucionando la eficiencia de las turbinas con la impresión metálica en 3D
Leer Más "Acerca de Met3DP
Actualización reciente
Nuestro producto
CONTACTO
¿Tiene alguna pregunta? ¡Envíenos un mensaje ahora! Atenderemos su solicitud con todo un equipo tras recibir su mensaje.

Polvos metálicos para impresión 3D y fabricación aditiva
PRODUCTO
cONTACT INFO
- Ciudad de Qingdao, Shandong, China
- [email protected]
- [email protected]
- +86 19116340731