Maßgeschneiderte Knochenplatten aus dem 3D-Druck von Titan
Inhaltsübersicht
Maßgefertigte Knochenplatten im 3D-Druckverfahren aus Titan: Eine neue Ära für orthopädische Lösungen
Der Bereich der orthopädischen Chirurgie befindet sich in einem tiefgreifenden Wandel, der durch Fortschritte in der Materialwissenschaft und der Fertigungstechnologie vorangetrieben wird. Zu den spannendsten Entwicklungen gehört die Anwendung der additiven Fertigung von Metallen, allgemein bekannt als 3D-Druckum patientenspezifische medizinische Implantate herzustellen. Dies ist besonders im Bereich der nicht-tragenden Knochenplatten von Bedeutung, wo die Individualisierung erhebliche Vorteile bietet. Unter Verwendung von Materialien wie der Titanlegierung Ti-6Al-4V ELI (Extra Low Interstitials) ermöglicht der 3D-Metalldruck die Herstellung individueller Knochenplatten mit komplizierten Geometrien, die perfekt auf die einzigartige Anatomie eines Patienten abgestimmt sind. Diese Technologie überwindet die Grenzen herkömmlicher Implantate, die nur eine Größe haben, und ebnet den Weg für bessere chirurgische Ergebnisse, kürzere Operationszeiten und eine schnellere Genesung des Patienten, insbesondere bei komplexen kraniofazialen, maxillofazialen und pädiatrischen Anwendungen. Für Lieferanten von Medizinprodukten, Händler und Beschaffungsabteilungen von Krankenhäusern ist es entscheidend, die Möglichkeiten und Feinheiten dieser Technologie zu verstehen, um an der Spitze der orthopädischen Versorgung zu bleiben. Unternehmen wie Met3dp leisten bei diesen Fortschritten Pionierarbeit und bieten umfassende Lösungen an, die von hochwertigen Metallpulvern bis hin zu fortschrittlichen Drucksystemen reichen und die Medizinindustrie in die Lage versetzen, die personalisierte Medizin zu übernehmen.

Einführung: Revolutionierung der Orthopädie mit maßgeschneiderten Titan-Knochenplatten
In der orthopädischen Chirurgie werden seit langem Knochenplatten zur Stabilisierung von Frakturen, zur Fusion von Gelenken und zur Rekonstruktion von Skelettdefekten eingesetzt. Traditionell werden diese Platten mit subtraktiven Methoden hergestellt, wobei in der Regel Standardformen und -größen aus Metallblöcken in medizinischer Qualität bearbeitet werden. Dieser Ansatz ist zwar für viele gängige Frakturen geeignet, greift aber oft zu kurz, wenn es um komplexe Anatomien oder besondere Patientenbedürfnisse geht, insbesondere in nicht-lasttragenden Situationen, in denen eine präzise Passform und Konturierung für eine optimale Funktion und Ästhetik von größter Bedeutung sind. Nicht lasttragende Platten werden typischerweise in Bereichen wie dem Schädel, dem Gesicht oder in bestimmten pädiatrischen Fällen verwendet, in denen die mechanischen Belastungen relativ gering sind, aber ein hoher Bedarf an anatomischer Genauigkeit besteht.
Das Aufkommen der additiven Fertigung von Metall (AM) oder des 3D-Drucks stellt einen Paradigmenwechsel bei der Entwicklung und Herstellung dieser wichtigen medizinischen Geräte dar. Anstatt Material abzutragen, werden bei der additiven Fertigung die Komponenten Schicht für Schicht direkt aus einem digitalen Modell aufgebaut, das in der Regel von einem CT- oder MRT-Scan des Patienten stammt. Dieses Verfahren ermöglicht Chirurgen und biomedizinischen Ingenieuren eine noch nie dagewesene Gestaltungsfreiheit bei der Herstellung von Knochenplatten, die wirklich patientenspezifisch sind.
Die Macht der Personalisierung:
Stellen Sie sich einen Patienten vor, der nach einem Trauma eine kraniofaziale Rekonstruktion benötigt. Bei der Verwendung herkömmlicher Methoden müsste eine Standardplatte während der Operation manuell gebogen werden - ein zeitaufwändiger Prozess, der stark von den Fähigkeiten des Chirurgen abhängt und zu einer suboptimalen Passform führen kann. Mit dem 3D-Metalldruck kann eine Platte virtuell entworfen werden, die die Konturen des Schädels des Patienten perfekt widerspiegelt, die notwendigen Schraubenlöcher an den idealen Stellen enthält und sogar Merkmale wie poröse Strukturen zur Förderung des Knochenwachstums (Osseointegration) integriert.
Exzellente Werkstoffe: Die Rolle des Titans:
Titanlegierungen, insbesondere Ti-6Al-4V ELI, sind die Materialien der Wahl für diese Anwendungen. Diese besondere Sorte bietet eine außergewöhnliche Kombination aus:
- Biokompatibilität: Es ist für den menschlichen Körper gut verträglich und minimiert das Risiko von Nebenwirkungen.
- Korrosionsbeständigkeit: Es widersteht der rauen Umgebung im Körper, ohne sich zu zersetzen.
- Verhältnis Stärke/Gewicht: Es sorgt für die nötige Stabilität, ohne übermäßig viel Platz oder Gewicht zu beanspruchen.
- Osseointegrationspotenzial: Oberflächenmodifikationen und poröse Strukturen, die mit AM möglich sind, können die Knochenintegration verbessern.
- MRI/CT-Kompatibilität: Es verursacht im Allgemeinen weniger Artefaktstörungen bei der medizinischen Bildgebung als einige andere Metalle.
Die Bezeichnung ‘ELI’ steht für Extra Low Interstitials (Sauerstoff, Stickstoff, Kohlenstoff, Eisen), was die Duktilität und Bruchzähigkeit des Materials erhöht - entscheidende Eigenschaften für die Sicherheit und Langlebigkeit von Implantaten.
Additive Fertigungstechnologien:
Für die Herstellung von Titan-Knochenplatten können verschiedene AM-Verfahren eingesetzt werden, wobei die Pulverbettfusion (Powder Bed Fusion, PBF) die gängigste Methode ist:
- Selektives Laserschmelzen (SLM) / Direktes Metall-Laser-Sintern (DMLS): Verwendet einen Hochleistungslaser zum selektiven Schmelzen von Bereichen eines Metallpulverbettes.
- Selektives Elektronenstrahlschmelzen (SEBM): Verwendet einen Elektronenstrahl in einer Vakuumumgebung zum Schmelzen des Pulvers. SEBM, wie die von Met3dp entwickelten Systeme, führt häufig zu geringeren Eigenspannungen und kann bestimmte Materialien effektiver verarbeiten, was es für die Herstellung medizinischer Implantate sehr geeignet macht.
Auswirkungen auf das Gesundheitswesen:
Die Auswirkungen von individuell gedruckten 3D-Knochenplatten sind weitreichend:
- Verbesserte chirurgische Ergebnisse: Eine bessere anatomische Passform führt zu einer stabileren Fixierung, einer potenziell schnelleren Heilung und besseren funktionellen und ästhetischen Ergebnissen.
- Reduzierte Betriebszeit: Vorgeformte, patientenspezifische Platten machen das intraoperative Biegen überflüssig und sparen wertvolle Zeit im Operationssaal.
- Komplexe Falllösungen: Ermöglicht eine effektive Behandlung von schwierigen Fällen mit einzigartigen anatomischen Variationen oder erheblichem Knochenverlust.
- Optimierung der Lieferkette: Durch die Möglichkeit der Herstellung auf Abruf müssen Krankenhäuser und Händler keine großen Bestände an Standardplatten in verschiedenen Größen vorhalten.
Als führendes Unternehmen auf dem Gebiet der additiven Fertigung von Metallen bietet Met3dp nicht nur hochmoderne SEBM-Druckanlagen, sondern auch sorgfältig ausgearbeitete Metallpulver wie Ti-6Al-4V ELI, die höchste Qualität und Konsistenz gewährleisten, die für kritische medizinische Anwendungen erforderlich sind. Unser umfassendes Fachwissen sowohl in der Materialwissenschaft als auch in den AM-Prozessen macht uns zu einem wichtigen Partner für Hersteller von Medizinprodukten, Großhandelslieferanten und Forschungseinrichtungen, die die transformative Kraft des 3D-Drucks in der Orthopädie nutzen möchten. Der Weg zu personalisierten, hochleistungsfähigen orthopädischen Implantaten wird immer schneller, und maßgeschneiderte Titan-Knochenplatten sind die Vorhut dieser Revolution.
Wofür werden nicht-lasttragende Knochenplatten verwendet? Anwendungen und Indikationen
Während lasttragende Implantate wie Hüftschäfte oder Wirbelsäulenfusionscages große Aufmerksamkeit erregen, spielen nicht lasttragende Knochenplatten eine ebenso wichtige Rolle bei der Wiederherstellung von Form und Funktion in bestimmten anatomischen Regionen. Diese Platten sind für die Fixierung in Bereichen vorgesehen, in denen die primären Kräfte relativ gering sind, und die Hauptziele sind anatomische Reduktion, Stabilisierung für die Heilung und Wiederherstellung der Kontur. Die Möglichkeit, diese Platten mithilfe des 3D-Metalldrucks individuell zu gestalten, bietet in diesen oft komplexen und heiklen chirurgischen Bereichen große Vorteile. Für Chirurgen, Entwickler von Medizinprodukten und Beschaffungsspezialisten in Krankenhäusern und Vertriebsnetzen ist es wichtig, die spezifischen Anwendungen zu verstehen.
Wichtigste Anwendungsbereiche:
- Kraniofaziale Chirurgie: Dies ist vielleicht der wichtigste Bereich für kundenspezifische nicht tragende Platten.
- Kranioplastik: Reparatur von Schädeldefekten, die durch Trauma, Tumorresektion oder dekompressive Kraniektomie entstanden sind. Eine präzise Passform ist entscheidend, um das Gehirn zu schützen und ein gutes kosmetisches Ergebnis zu erzielen. der 3D-Druck ermöglicht große, komplexe Platten, die sich perfekt an die Konturen des Schädeldefekts des Patienten anpassen und häufig Merkmale wie integrierte Flansche für die Schraubenbefestigung oder poröse Oberflächen zur Förderung der Gewebsintegration aufweisen.
- Orbitalboden-Rekonstruktion: Reparatur von Frakturen der dünnen Knochen, die die Augenhöhle umgeben. Genauigkeit ist entscheidend, um die Position und Bewegung des Auges wiederherzustellen und Komplikationen wie Doppelbilder (Diplopie) oder ein eingesunkenes Auge (Enophthalmus) zu vermeiden. Maßgefertigte Platten können die komplexe 3D-Form des Augenhöhlenbodens präzise nachbilden.
- Rekonstruktion des Unterkiefers (Kiefers): Während der Unterkiefer beim Kauen erheblichen Kräften ausgesetzt ist, können bestimmte rekonstruktive Platten, insbesondere solche, die zur Überbrückung von Defekten nach einer Tumorentfernung oder einem Trauma an bestimmten Stellen oder zur Steuerung der Knochenregeneration verwendet werden, in erster Linie nicht tragend sein oder zur vorübergehenden Stabilisierung vor einer endgültigen, tragenden Rekonstruktion dienen. Die individuelle Anpassung gewährleistet die richtige Ausrichtung und Kontur.
- Rekonstruktion des Mittelgesichts (z. B. Le-Fort-Frakturen): Stabilisierung komplexer Frakturen des Oberkiefers und der umgebenden Gesichtsknochen. Die anatomische Reposition ist der Schlüssel zur Wiederherstellung der Gesichtsstruktur und der Okklusion (Biss).
- Kiefer- und Gesichtschirurgie: Sie überschneidet sich weitgehend mit der kraniofazialen Chirurgie und umfasst insbesondere Eingriffe im Kiefer- und Gesichtsbereich.
- Orthognatische Chirurgie: Korrektive Kieferchirurgie zur Behebung von Zahnfehlstellungen und Gesichtsasymmetrien. Da die Fixierung stabil sein muss, sind die verwendeten Platten oft relativ klein und so geformt, dass sie zu den reponierten Knochensegmenten passen. Individuelle Führungen und Platten können die Präzision erhöhen.
- Jochbeinfrakturen (Wangenknochen): Wiederherstellung der Prominenz und Symmetrie des Wangenknochens.
- Neurochirurgie: In erster Linie für kranioplastische Anwendungen, die eine nahtlose Integration in neurochirurgische Verfahren gewährleisten. Die Fähigkeit, dünne und dennoch stabile Platten herzustellen, die sich perfekt an den Schädeldefekt anpassen, ist von großem Vorteil.
- Pädiatrische Orthopädie: Die Knochen von Kindern befinden sich noch im Wachstum, und anatomische Abweichungen sind üblich.
- Korrektive Osteotomien: Chirurgische Schnitte im Knochen zur Korrektur von Deformitäten (z. B. an den oberen Gliedmaßen oder am Schädel). Bei bestimmten Anwendungen mit geringer Belastung können maßgefertigte Platten eine stabile, auf die einzigartige Anatomie des Kindes zugeschnittene Fixierung bieten, ohne die Wachstumsplatten zu beeinträchtigen, wenn sie sorgfältig entworfen werden.
- Korrektur von angeborenen Fehlbildungen: Behandlung von Skelettanomalien, die bei der Geburt vorhanden sind, insbesondere im kraniofazialen Bereich.
- Hand- und Fußchirurgie: Fixierung von Frakturen oder Fusionen kleiner Knochen in Fingern, Handgelenk, Zehen und Fuß. Obwohl diese Knochen Kräften ausgesetzt sind, dienen viele der verwendeten Platten in erster Linie der Stabilisierung während der Heilung und sind nicht den hohen Belastungen ausgesetzt, die bei großen Röhrenknochen auftreten. Eine individuelle Anpassung kann bei komplexen Frakturen oder ungewöhnlichen Anatomien von Vorteil sein.
Warum die Anpassung in diesen Bereichen wichtig ist:
- Komplexe Anatomie: Das kraniofaziale Skelett, die Hände und die Füße haben komplizierte dreidimensionale Strukturen. Standardplatten müssen intraoperativ oft stark gebogen werden, was die Platte schwächen und die Passform beeinträchtigen kann.
- Ästhetik: Bei der Gesichtsrekonstruktion ist die Wiederherstellung der Symmetrie und der natürlichen Konturen entscheidend für die Lebensqualität des Patienten. Maßgefertigte Platten bieten hervorragende kosmetische Ergebnisse.
- Dünne Abdeckung des Weichgewebes: In Bereichen wie der Kopfhaut oder dem Gesicht können sperrige oder schlecht sitzende Platten spürbar oder sichtbar sein oder sogar zu Hautverletzungen führen. Niedrigprofilige, präzise konturierte individuelle Platten minimieren diese Risiken.
- Reduzierte Operationszeit: Wie bereits erwähnt, wird durch den Wegfall des manuellen Biegens der Platte die Operationsdauer erheblich verkürzt, was die Anästhesiezeit und mögliche Komplikationen reduziert.
- Verbesserte Stabilität: Ein perfekter Sitz verteilt die Belastung gleichmäßiger und sorgt für eine sicherere Fixierung, was eine zuverlässige Heilung fördert.
Überlegungen für Lieferanten und Vertriebshändler:
Die Verlagerung hin zu maßgeschneiderten Implantaten hat Auswirkungen auf das traditionelle Lieferkettenmodell. Während ein Vorrat an Standardplatten für Notfalltraumata weiterhin notwendig ist, wächst die Nachfrage von Krankenhäusern und chirurgischen Zentren nach zuverlässigen Partnern, die den gesamten Arbeitsablauf für individuelle Implantate erleichtern können:
- Bildakquisition & Segmentierung: Protokolle für die Gewinnung hochwertiger CT/MRI-Scans und deren genaue Umwandlung in 3D-Modelle.
- Zusammenarbeit bei der Gestaltung: Plattformen oder Dienste, die es Chirurgen ermöglichen, mit Ingenieuren bei der Entwicklung von Implantaten zusammenzuarbeiten.
- Kompetenz in der Fertigung: Zugang zu validierten Metall-3D-Druckverfahren (wie SEBM oder SLM) unter Verwendung zertifizierter medizinischer Materialien (wie Ti-6Al-4V ELI). Unternehmen wie Met3dp mit ihrem integrierten Ansatz, der fortschrittlicher Pulverherstellung und Drucksysteme, sind hier entscheidend.
- Qualitätssicherung: Strenge Inspektions- und Dokumentationsverfahren, die den gesetzlichen Normen entsprechen (z. B. ISO 13485).
- Verwaltung der Vorlaufzeit: Effiziente Prozesse für die Lieferung maßgeschneiderter Implantate innerhalb klinisch akzeptabler Zeiträume.
Die Anwendungen für nicht-tragende, individuell gedruckte 3D-Knochenplatten sind vielfältig und expandieren. Sie stellen einen bedeutenden Fortschritt in der personalisierten Chirurgie dar und bieten greifbare Vorteile in komplexen anatomischen Regionen, in denen Präzision, Passform und Ästhetik von größter Bedeutung sind. Für Gesundheitsdienstleister und ihre Beschaffungspartner bedeutet die Nutzung dieser Technologie, dass sie mit kompetenten Herstellern und Lieferanten zusammenarbeiten müssen, die in der Lage sind, diese anspruchsvollen, patientenspezifischen Lösungen zu liefern.

Warum 3D-Metalldruck für individuelle Knochenplatten verwenden? Die wichtigsten Vorteile für Patienten und Leistungserbringer
Die Anwendung der additiven Fertigung (AM) von Metall für die Herstellung von nicht-tragenden Knochenplatten, insbesondere von patientenspezifischen Platten, ist nicht nur ein neuartiger Ansatz, sondern bietet Patienten, Chirurgen, Krankenhäusern und Medizinproduktehändlern gleichermaßen eine Reihe von Vorteilen gegenüber herkömmlichen Fertigungsmethoden. Herkömmliche Verfahren wie CNC-Bearbeitung oder Guss haben der Orthopädie zwar jahrzehntelang gute Dienste geleistet, aber sie schränken die Komplexität des Designs und die Möglichkeiten der individuellen Anpassung von vornherein ein. Der 3D-Metalldruck überwindet diese Einschränkungen und führt zu erheblichen Verbesserungen bei den klinischen Ergebnissen, der betrieblichen Effizienz und dem Lieferkettenmanagement.
1. Unübertroffene patientenindividuelle anatomische Passform:
- Der Kernnutzen: Dies ist der wichtigste Vorteil. Anhand der Daten aus einem CT- oder MRT-Scan des Patienten wird ein 3D-Modell des betroffenen Knochens erstellt. Die Knochenplatte wird dann digital so gestaltet, dass sie perfekt an die einzigartigen Konturen und die Geometrie der Anatomie des jeweiligen Patienten angepasst ist.
- Klinische Auswirkungen:
- Verbesserte Stabilität: Eine perfekt sitzende Platte verteilt die Belastung gleichmäßiger auf die Knochen-Implantat-Grenzfläche, was zu einer stabileren Fixierung führt und das Risiko von Mikrobewegungen, die die Heilung behindern können, verringert.
- Verringertes Risiko von Malunion/Nichtunion: Eine präzise anatomische Reposition, die durch eine maßgefertigte Platte ermöglicht wird, verringert das Risiko, dass der Knochen in einer falschen Position einheilt (Malunion) oder gar nicht einheilt (Nonunion).
- Bessere funktionale Ergebnisse: In Bereichen wie dem Kiefer oder der Augenhöhle ist die präzise Wiederherstellung der Anatomie entscheidend für die Funktion (z. B. korrekter Biss, normale Augenbewegung).
- Überlegene Ästhetik: Bei der kraniofazialen Rekonstruktion stellt eine perfekt konturierte Platte das natürliche Aussehen viel besser wieder her als eine gebogene Standardplatte.
- Traditionelle Einschränkungen: Standardplatten gibt es in vorgegebenen Größen und Formen. Chirurgen müssen diese Platten während des Eingriffs oft ausgiebig biegen, um sie der Anatomie des Patienten anzunähern. Dieser Biegeprozess ist zeitaufwändig, hängt stark von der Erfahrung des Chirurgen ab, kann die Platte schwächen und führt möglicherweise nie zu einer wirklich perfekten Passform.
2. Gestaltungsfreiheit für komplexe Geometrien:
- Jenseits einfacher Formen: AM baut Teile Schicht für Schicht auf und ermöglicht so die Herstellung hochkomplexer Formen, die mit herkömmlichen Methoden nur schwer oder gar nicht zu realisieren sind.
- Klinische Anwendungen:
- Integrierte poröse Strukturen: Die Platten können mit Gitter- oder Trabekelstrukturen auf der knochenberührenden Oberfläche oder im gesamten Plattenkörper (sofern strukturell geeignet) gestaltet werden. Diese Poren ahmen die Struktur der Spongiosa nach und fördern die Osseointegration - das direkte Einwachsen des Knochens in das Implantat - was zu einer besseren langfristigen Stabilität und biologischen Fixierung führt.
- Optimierte Formen: Die Platten können mit variabler Dicke, internen Kanälen oder einzigartigen Konturen gestaltet werden, um empfindliche anatomische Strukturen zu vermeiden, spezifische Schraubentrajektorien zu berücksichtigen oder das Gesamtvolumen und Gewicht des Implantats zu minimieren und gleichzeitig die erforderliche Festigkeit zu erhalten.
- Konsolidierte Komponenten: Funktionen, für die bei einer herkömmlichen Montage mehrere Teile erforderlich wären, können möglicherweise in eine einzige 3D-gedruckte Komponente integriert werden.
- Traditionelle Einschränkungen: Die maschinelle Bearbeitung ist durch die Zugänglichkeit des Werkzeugs begrenzt, was interne Kanäle oder komplexe poröse Netze extrem schwierig macht. Beim Gießen gibt es Einschränkungen in Bezug auf die erreichbaren Details und die Kontrolle der Porosität.
3. Geringere chirurgische Zeit und Komplexität:
- Beseitigung des intraoperativen Biegens: Da die Platte entsprechend der spezifischen Anatomie des Patienten vorkonturiert geliefert wird, entfällt der zeitaufwändige und oft schwierige Schritt des Biegens der Platte während der Operation oder wird erheblich reduziert.
- Klinische Auswirkungen:
- Kürzere Dauer der Anästhesie: Eine kürzere Operationszeit bedeutet eine kürzere Narkosezeit für den Patienten, was die damit verbundenen Risiken verringert.
- Geringerer Blutverlust: Kürzere Verfahren korrelieren in der Regel mit einem geringeren intraoperativen Blutverlust.
- Gesteigerte OP-Effizienz: Schnellere Durchlaufzeiten verbessern die Auslastung des Operationssaals, was der Krankenhauslogistik zugute kommt und die Wartelisten verkürzen kann.
- Geringere Ermüdung des Chirurgen: Eine Vereinfachung des Verfahrens kann die Ermüdung des Chirurgen verringern und so seine Konzentration und Leistungsfähigkeit verbessern.
- Planungsinstrumente: Der digitale Entwurfsprozess umfasst häufig eine chirurgische Planungssoftware, die es den Chirurgen ermöglicht, den Eingriff zu visualisieren, die Platzierung der Schrauben zu planen und Herausforderungen vorauszusehen, bevor sie den Operationssaal betreten. Neben der Platte können auch benutzerdefinierte Bohrschablonen in 3D gedruckt werden, um eine genaue Platzierung zu gewährleisten.
4. Materialeffizienz und Abfallvermeidung:
- Additiv vs. Subtraktiv: Bei AM werden Teile nur dort hergestellt, wo sie benötigt werden. Im Gegensatz dazu beginnt die traditionelle Bearbeitung mit einem größeren Materialblock und entfernt (subtrahiert) überschüssiges Material, um die endgültige Form zu erreichen.
- Auswirkungen:
- Weniger Materialverbrauch: Insbesondere bei teuren medizinischen Legierungen wie Ti-6Al-4V ELI führt die Reduzierung des Materialabfalls zu Kosteneinsparungen und einer besseren Ressourcennutzung. Während ungenutztes Pulver in PBF-Prozessen oft recycelt werden kann, ist das gesamte "Buy-to-Fly"-Verhältnis (Verhältnis zwischen dem Gewicht des Rohmaterials und dem Gewicht des fertigen Teils) bei AM im Allgemeinen viel besser als bei der Bearbeitung komplexer Teile.
- Nachhaltigkeit: Weniger Abfall trägt zu ökologisch nachhaltigeren Herstellungsverfahren bei.
5. Verbessertes Management von Lieferkette und Beständen:
- Produktion auf Abruf: Maßgefertigte Platten werden speziell für jeden Patienten hergestellt, so dass Krankenhäuser und Händler keine großen Bestände an Standardplatten in zahlreichen Größen und Konfigurationen mehr vorhalten müssen.
- Auswirkungen für Anbieter und Vertreiber:
- Geringere Lagerkosten: Geringere Kapitalbindung im Lager, geringerer Lagerplatzbedarf und geringeres Risiko des Verfalls oder der Veralterung von Implantaten.
- Rationalisierte Logistik: Vereinfachtes Bestellverfahren, das sich an den Bedürfnissen der Patienten orientiert und nicht an der Masse der Standardteile.
- Potenzial für Point-of-Care: Die langfristige Vision umfasst die Möglichkeit, dass Krankenhäuser über eigene oder regionale AM-Einrichtungen verfügen, die eine schnellere Herstellung von maßgeschneiderten Implantaten ermöglichen und die Lieferkette weiter revolutionieren. Zuverlässig Anbieter von 3D-Metalldruckdiensten wie Met3dp sind wichtige Partner bei der Realisierung dieses Potenzials, sei es durch eine zentrale Produktion oder die Unterstützung lokaler Einrichtungen.
- Traditionelles Modell: Dies erfordert umfangreiche Prognosen, Großeinkäufe und eine komplexe Bestandsverwaltung durch Händler und Krankenhäuser, um die Verfügbarkeit geeigneter Standardimplantatgrößen zu gewährleisten.
6. Erleichterung der Innovation:
- Rapid Prototyping: AM ermöglicht eine schnelle Iteration neuer Implantatdesigns. Ingenieure und Chirurgen können neue Konzepte für nicht-tragende Platten schnell testen und verfeinern und so das Innovationstempo bei orthopädischen Behandlungen beschleunigen.
- Neuartige Lösungen: Die gestalterische Freiheit ermöglicht völlig neue Ansätze zur Fixierung und Rekonstruktion, die bisher nicht denkbar waren.
Zusammenfassende Tabelle: AM vs. traditionelle Herstellung von Knochenplatten
| Merkmal | Additive Fertigung von Metall (3D-Druck) | Traditionelle Fertigung (Bearbeitung/Gießen) |
|---|---|---|
| Personalisierung | Patientenspezifisch, hohe anatomische Übereinstimmung | Standardgrößen, erfordert manuelles Biegen |
| Geometrische Komplexität | Hoch (poröse Strukturen, interne Kanäle, komplexe Kurven) | Begrenzt durch Werkzeug-/Gussformbeschränkungen |
| Anatomische Passform | Ausgezeichnet, vorkonturiert | Ungefähr, beruht auf intraoperativem Biegen |
| Intraoperative Zeit | Reduziert (kein/weniger Biegen) | Erhöht (erfordert Bücken) |
| Osseointegration | Potenzial für entworfene poröse Strukturen | Begrenzt (Oberflächenbehandlungen möglich) |
| Materialabfälle | Niedriger (additives Verfahren) | Höher (subtraktives Verfahren) |
| Bestandsaufnahme Modell | Abrufbare, reduzierte Bestände | Erfordert einen großen Bestand an Standardgrößen |
| Entwurf Iteration | Schnell, erleichtert das Rapid Prototyping | Langsamer, teurer |
| Vorlaufzeit (kundenspezifisch) | Tage bis Wochen (prozessabhängig) | Nicht zutreffend (Standardteile leicht erhältlich) |
| Vorlaufzeit (Standard) | Nicht zutreffend (kann bei Bedarf Standard herstellen) | Sofort (wenn vorrätig) |
In Blätter exportieren
Zusammenfassend lässt sich sagen, dass der 3D-Metalldruck, insbesondere unter Verwendung fortschrittlicher Systeme und hochwertiger Materialien von Anbietern wie Met3dp, eine Kaskade von Vorteilen für die Herstellung individueller, nicht-lasttragender Knochenplatten bietet. Vom grundlegenden Vorteil einer perfekten Passform für den Patienten, die zu besseren klinischen Ergebnissen führt, bis hin zu betrieblicher Effizienz im OP und optimierten Lieferketten für Großhändler und Krankenhäuser, stellt AM einen überlegenen technologischen Ansatz für diese anspruchsvollen orthopädischen Anwendungen dar.
Ti-6Al-4V ELI: Das Goldstandardmaterial für 3D-gedruckte Knochenplatten
Der Erfolg eines jeden medizinischen Implantats hängt entscheidend von dem Material ab, aus dem es hergestellt wird. Bei 3D-gedruckten nicht-tragenden Knochenplatten ist das Material der Wahl ganz klar Ti-6Al-4V ELI (Güte 23). Diese spezielle Titanlegierung hat sich ihren Ruf als Goldstandard aufgrund einer außergewöhnlichen Kombination von Eigenschaften erworben, die sie einzigartig für die langfristige Implantation im menschlichen Körper und für die Kompatibilität mit additiven Fertigungsverfahren machen. Um zu verstehen, warum Ti-6Al-4V ELI bevorzugt wird, muss man sich mit seiner Zusammensetzung, seinen mechanischen Eigenschaften, seiner Biokompatibilität und den spezifischen Anforderungen an Metallpulver für den 3D-Druck beschäftigen.
Zusammensetzung und die Bedeutung von ‘ELI’:
Ti-6Al-4V, auch bekannt als Titan Grad 5, ist eine Alpha-Beta-Titanlegierung. Seine nominale Zusammensetzung ist:
- Titan (Ti): Grundelement
- Aluminium (Al): ~6% (Alpha-Phasen-Stabilisator, erhöht die Festigkeit)
- Vanadium (V): ~4% (Beta-Phasen-Stabilisator, erhöht die Festigkeit)
Die Bezeichnung “ELI” steht für Extra niedrige Interstitials. Das bedeutet, dass die maximal zulässigen Grenzwerte für Zwischengitterelemente - hauptsächlich Sauerstoff (O), Stickstoff (N), Kohlenstoff (C) und Eisen (Fe) - deutlich niedriger sind als bei Standard-Titan Grad 5.
- Standard Ti-6Al-4V (Grad 5): Erlaubt in der Regel höhere Sauerstoffgehalte (z. B. ≤ 0,20 %).
- Ti-6Al-4V ELI (Güte 23): Vorgeschrieben sind niedrigere Werte (z. B. Sauerstoff ≤ 0,13 %).
Warum sind geringe Zwischenräume für medizinische Implantate so wichtig?
Zwischengitterelemente, selbst in kleinen Mengen, können die mechanischen Eigenschaften der Legierung erheblich beeinflussen. Höhere Werte erhöhen in der Regel die Festigkeit, verringern aber die Duktilität (die Fähigkeit, sich zu verformen, ohne zu brechen) und die Bruchzähigkeit (Widerstand gegen Rissausbreitung). Für ein medizinisches Implantat wie eine Knochenplatte, insbesondere eine mit komplexer Geometrie, die im 3D-Druckverfahren hergestellt wird, duktilität und Bruchzähigkeit sind für die Sicherheit und langfristige Leistungsfähigkeit von größter Bedeutung. Durch die Verringerung des interstitiellen Anteils ist Ti-6Al-4V ELI nachsichtiger, weniger spröde und widerstandsfähiger gegen Ermüdungsbrüche im Vergleich zu Standard Grade 5, was es zur bevorzugten Wahl für kritische Anwendungen wie chirurgische Implantate macht.
Wichtige Eigenschaften von Ti-6Al-4V ELI für Knochenplatten:
- Ausgezeichnete Biokompatibilität: Dies ist für jedes Implantatmaterial unverzichtbar.
- Unbeweglichkeit: Titan bildet von Natur aus eine stabile, dünne und fest haftende Oxidschicht (TiO2) auf seiner Oberfläche, sobald es der Luft oder Körperflüssigkeiten ausgesetzt wird. Diese Schicht ist in der salzhaltigen Umgebung des Körpers äußerst korrosionsbeständig und verhindert die Auslaugung von Metallionen in das umliegende Gewebe.
- Geringe Toxizität: Titan und seine üblichen Legierungselemente (Aluminium und Vanadium in diesen Mengen) haben eine ausgezeichnete Biokompatibilität mit minimalen unerwünschten Gewebereaktionen oder systemischer Toxizität gezeigt.
- Osseointegration: Obwohl Titan von Natur aus bioinert ist, können sich an seiner Oberfläche leicht Knochenzellen (Osteoblasten) anlagern und vermehren, was zu einer direkten Anlagerung von Knochen an die Implantatoberfläche führt - ein Prozess, der Osseointegration genannt wird. Dieser Prozess wird als Osseointegration bezeichnet und ist für die langfristige Stabilität von orthopädischen Implantaten von entscheidender Bedeutung. der 3D-Druck ermöglicht die gezielte Gestaltung poröser Oberflächen, die diese biologische Fixierung weiter verbessern.
- Hohes Verhältnis von Festigkeit zu Gewicht:
- Titanlegierungen sind wesentlich leichter als rostfreie Stähle oder Kobalt-Chrom-Legierungen (andere gängige Implantatmaterialien), bieten aber eine vergleichbare oder höhere Festigkeit.
- Dies führt zu einer starken, stabilen Fixierung ohne übermäßiges Gewicht oder Volumen, was besonders bei kraniofazialen Anwendungen wichtig ist, bei denen ein möglichst geringes Implantatprofil erwünscht ist.
- Ausgezeichnete Korrosionsbeständigkeit:
- Die stabile TiO2-Passivschicht bietet eine außergewöhnliche Beständigkeit gegen Korrosion und Abbau in der physiologischen Umgebung und gewährleistet die strukturelle Integrität des Implantats über die vorgesehene Lebensdauer.
- Geeignete mechanische Eigenschaften:
- Elastizitätsmodul: Ti-6Al-4V ELI hat einen Elastizitätsmodul (Steifigkeit) von etwa 110-115 GPa. Er ist zwar immer noch steifer als natürlicher Knochen (typischerweise 10-30 GPa), aber deutlich niedriger als Edelstahl (~200 GPa) oder CoCr-Legierungen (~240 GPa). Dieser niedrigere Modul kann die Stressabschirmung reduzieren - ein Phänomen, bei dem ein sehr steifes Implantat zu viel Last trägt und den angrenzenden Knochen vor normalen physiologischen Belastungen abschirmt, was im Laufe der Zeit zu Knochenresorption (Schwächung) führen kann. Während dies bei nicht-tragenden Platten weniger kritisch ist als bei Hüftschäften, ist eine engere Übereinstimmung mit dem Knochenmodul im Allgemeinen vorteilhaft.
- Ermüdungsfestigkeit: Implantate sind zyklischen Belastungen ausgesetzt, auch in nicht belasteten Bereichen (z. B. durch Muskelzerrungen, leichte Stöße). Ti-6Al-4V ELI weist eine ausgezeichnete Ermüdungsfestigkeit auf, die eine lange Lebensdauer unter physiologischen Bedingungen gewährleistet. Die erhöhte Bruchzähigkeit der Sorte ELI trägt ebenfalls zur Ermüdungsfestigkeit bei.
- Zugfestigkeit: Besitzt eine hohe Zugfestigkeit (typischerweise >830 MPa im geglühten Zustand), um funktionalen Belastungen ohne bleibende Verformung standzuhalten.
- MRI- und CT-Kompatibilität:
- Titan ist nicht ferromagnetisch und daher für Patienten, die sich einer MRT-Untersuchung unterziehen, sicher. Es erzeugt zwar einige Artefakte (Verzerrungen) in MRT- und CT-Bildern, doch sind diese in der Regel weniger schwerwiegend als bei Edelstahl- oder CoCr-Legierungen, was eine bessere postoperative Darstellung des umliegenden Gewebes ermöglicht.
Pulveranforderungen für die additive Fertigung:
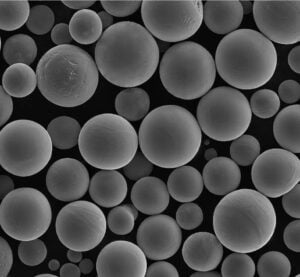
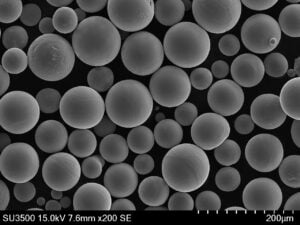
Die Herstellung hochwertiger Ti-6Al-4V ELI-Knochenplatten mittels AM erfordert zunächst ein Metallpulver, das strengen Spezifikationen entspricht. Die Eigenschaften des Pulvers haben einen direkten Einfluss auf die Stabilität des Druckprozesses und die Dichte, die Mikrostruktur und die mechanischen Eigenschaften des fertigen Teils. Die wichtigsten Eigenschaften des Pulvers sind:
- Sphärizität: Die Pulverpartikel sollten idealerweise sehr kugelförmig sein. Dies fördert eine gute Fließfähigkeit des Pulvers (die Fähigkeit des Pulvers, sich gleichmäßig über die Bauplattform zu verteilen) und gewährleistet eine gleichmäßige Packungsdichte, was zu einem gleichmäßigen Schmelzen und vollständig dichten Endteilen führt.
- Partikelgrößenverteilung (PSD): Der Bereich und die Verteilung der Partikelgrößen müssen sorgfältig kontrolliert und für den jeweiligen AM-Prozess (z. B. SLM oder SEBM) optimiert werden. Eine geeignete PSD gewährleistet eine gute Pulverbettdichte und ein effizientes Schmelzen. Typische Bereiche sind 15-53 µm oder 45-105 µm, je nach Maschine und Anwendung.
- Fließfähigkeit: Wie bereits erwähnt, ist eine gute Fließfähigkeit wichtig, um das Pulverbett gleichmäßig Schicht für Schicht aufzutragen. Eine schlechte Fließfähigkeit kann zu Defekten wie Porosität führen.
- Chemische Reinheit: Das Pulver muss die Normen für die chemische Zusammensetzung von Ti-6Al-4V ELI (z. B. ASTM F136) streng einhalten und darf nur minimale Verunreinigungen enthalten. Die Sauerstoffaufnahme bei der Herstellung und Handhabung des Pulvers muss streng kontrolliert werden.
- Fehlen von Satelliten und innere Porosität: Eine qualitativ hochwertige Pulverproduktion zielt darauf ab, kleinere Partikel, die an größeren Partikeln haften (Satelliten), und interne Gasporen innerhalb der Pulverpartikel zu minimieren, die sich beide negativ auf die Verarbeitung und die Qualität des Endprodukts auswirken können.
Met3dp’s Expertise in der Pulverproduktion:
Für Hersteller von Medizinprodukten und Großhandelslieferanten ist es von entscheidender Bedeutung, eine gleichbleibende Versorgung mit hochwertigem Ti-6Al-4V ELI-Pulver sicherzustellen, das diese anspruchsvollen Kriterien erfüllt. Hier spielen spezialisierte Pulverhersteller wie Met3dp eine wichtige Rolle.
- Fortgeschrittene Zerstäubungstechniken: Met3dp verwendet die branchenführenden Technologien der Gaszerstäubung (GA) und des Plasma-Rotations-Elektroden-Verfahrens (PREP).
- Gaszerstäubung: Verwendet Hochdruck-Inertgasstrahlen, um einen Strom von geschmolzenem Ti-6Al-4V ELI in feine Tröpfchen aufzubrechen, die zu kugelförmigem Pulver erstarren. Met3dp verwendet einzigartige Düsen- und Gasströmungsdesigns, um die Sphärizität und Fließfähigkeit zu optimieren und gleichzeitig die Kontamination zu minimieren.
- VORBEREITEN: Rotiert eine Abschmelzelektrode der gewünschten Legierung mit hoher Geschwindigkeit in einer inerten Atmosphäre. Ein Plasmabrenner schmilzt die Spitze der Elektrode auf, und die Zentrifugalkraft schleudert geschmolzene Tröpfchen aus, die sich im Flug verfestigen und in der Regel hochgradig kugelförmige Pulver mit sehr wenigen Satelliten oder inneren Porositäten ergeben - ideal für kritische Anwendungen.
- Qualitätskontrolle: Strenge Qualitätskontrollmaßnahmen, einschließlich chemischer Analyse, PSD-Messung, Morphologiebeurteilung (z. B. mittels Rasterelektronenmikroskopie) und Fließfähigkeitsprüfung, stellen sicher, dass jede Charge von Met3dp’s Ti-6Al-4V ELI-Pulver den Spezifikationen entspricht, die für anspruchsvolle 3D-Druckanwendungen aus Metallinsbesondere im medizinischen Bereich.
Vergleichstabelle: Ti-6Al-4V ELI vs. andere Implantatmaterialien
| Eigentum | Ti-6Al-4V ELI (Güte 23) | 316L-Edelstahl | CoCr-Legierung (ASTM F75) | PEEK (Polymer) |
|---|---|---|---|---|
| Primäre Verwendung | Orthopädie, Zahnmedizin | Orthopädie (Trauma) | Orthopädie (Gelenke) | Wirbelsäule, kraniofazial |
| Biokompatibilität | Ausgezeichnet | Gut | Gut | Ausgezeichnet |
| Dichte (g/cm³) | ~4.43 | ~8.0 | ~8.3-9.2 | ~1.3 |
| Elastizitätsmodul (GPa) | ~114 | ~193 | ~210-240 | ~3.6 |
| Zugfestigkeit (MPa) | >830 | >515 | >655 | ~100 |
| Korrosionsbeständigkeit | Ausgezeichnet | Gut (Lochfraßgefahr) | Sehr gut | Ausgezeichnet |
| MRI-Kompatibilität | Gut (nicht ferromagnetisch) | Schlecht (ferromagnetisch) | Gerecht (paramagnetisch) | Ausgezeichnet |
| Osseointegration | Gut (Verstärkt durch Poren) | Schlecht | Messe | Schlecht (Bioinert) |
| 3D-Druckbarkeit | Ausgezeichnet | Gut | Gut | Möglich (FDM/SLS) |
In Blätter exportieren
Zusammenfassend lässt sich sagen, dass Ti-6Al-4V ELI aufgrund seiner einzigartigen Mischung aus Biokompatibilität, mechanischer Festigkeit, Duktilität, Bruchzähigkeit, Korrosionsbeständigkeit und Eignung für fortschrittliche Fertigungsverfahren wie SEBM und SLM das Material der Wahl für 3D-gedruckte nichttragende Knochenplatten ist. Die strenge Kontrolle der interstitiellen Elemente in der ELI-Sorte bietet eine zusätzliche Sicherheitsmarge, die für medizinische Implantate entscheidend ist. Der Zugang zu hochwertigem, kontaminationsfreiem Pulver mit optimalen Eigenschaften, wie es Met3dp mit Hilfe fortschrittlicher Zerstäubungstechniken herstellt, ist von grundlegender Bedeutung, um das volle Potenzial dieses Materials bei der Entwicklung von patientenspezifischen orthopädischen Lösungen der nächsten Generation auszuschöpfen. Quellen und zugehörige Inhalte
Designüberlegungen für additiv gefertigte Knochenplatten: Optimierung von Passform und Funktion
Der bemerkenswerte Vorteil der additiven Fertigung von Metallen (AM) liegt in ihrer Fähigkeit, patientenspezifische Knochenplatten mit beispielloser anatomischer Konformität und potenziell verbesserter biologischer Funktion herzustellen. Um dieses Potenzial zu erschließen, ist jedoch mehr als nur der Zugang zu einem 3D-Drucker erforderlich. Es bedarf eines durchdachten und ausgefeilten Designansatzes, insbesondere des Design for Additive Manufacturing (DfAM). Das Design einer kundenspezifischen 3D-gedruckten Knochenplatte ist ein iterativer, gemeinschaftlicher Prozess, der medizinische Bilddaten, biomechanische Prinzipien, chirurgische Anforderungen und die spezifischen Fähigkeiten und Einschränkungen der gewählten AM-Technologie (wie SLM oder SEBM) und des Materials (Ti-6Al-4V ELI) integriert. Für Medizintechniker, Chirurgen und die Beschaffungsteams, die diese kundenspezifischen Lösungen beschaffen, ist das Verständnis dieser Designüberlegungen von größter Bedeutung.
1. High-Fidelity-Eingangsdaten: Die Grundlage
Der gesamte Prozess beginnt mit der Erfassung hochwertiger medizinischer Bilddaten, in der Regel Computertomographie (CT)-Scans der relevanten Anatomie des Patienten.
- Scan-Qualität: Die Auflösung, die Schichtdicke und die Gesamtqualität des CT-Scans wirken sich direkt auf die Genauigkeit des resultierenden 3D-Modells und folglich auf die Passform des endgültigen Implantats aus. Standardisierte Protokolle für Implantatplanungs-Scans sind unerlässlich. Es können auch MRT-Scans verwendet werden, insbesondere zur Visualisierung von Weichgewebe, aber die CT liefert im Allgemeinen eine bessere Knochendefinition.
- Bildsegmentierung: Spezialisierte Software wird verwendet, um die 2D-Scandaten (DICOM-Format) in ein anatomisches 3D-Modell (oft in STL oder ähnlichen Formaten) umzuwandeln. Dieser entscheidende Schritt, die so genannte Segmentierung, umfasst die Identifizierung und Isolierung des/der interessierenden Knochens/Knochen von den umgebenden Geweben. Die Genauigkeit ist hier entscheidend und erfordert oft erfahrene Techniker oder KI-gestützte Tools. Fehler bei der Segmentierung schlagen sich direkt in Ungenauigkeiten beim Implantatdesign nieder.
- Integrität der Daten: Die Gewährleistung des Datenschutzes und der Sicherheit von Patientendaten in diesem digitalen Arbeitsablauf ist nicht verhandelbar, da Vorschriften wie HIPAA oder GDPR eingehalten werden müssen.
2. Anatomische Konformität und Plattenkonturierung:
- Virtuelle Anprobe: Die Hauptaufgabe beim Design besteht darin, die Geometrie der Platte so zu gestalten, dass sie perfekt mit dem 3D-Modell der Knochenoberfläche des Patienten übereinstimmt. Mit Hilfe von CAD-Software (Computer-Aided Design) können die Designer die Platte virtuell auf das Knochenmodell drapieren oder modellieren.
- Plattengrenzen und Mächtigkeit: Die Ausdehnung der Platte (wie weit sie reicht) und ihr Dickenprofil sind entscheidend. Die Dicke kann entlang der Platte variiert werden - möglicherweise dicker über der Frakturstelle oder dem Defekt und dünner an den Rändern, um die Tastbarkeit unter dünner Haut zu minimieren (insbesondere im kraniofazialen Bereich). DfAM ermöglicht weiche Übergänge und organische Formen, die Spannungskonzentrationen minimieren.
- Kritische Strukturen meiden: Das Design muss sorgfältig auf nahe gelegene Nerven, Blutgefäße, Zahnwurzeln oder andere empfindliche anatomische Strukturen Rücksicht nehmen und sicherstellen, dass die Platte und die Schrauben nicht auf diese stoßen.
3. Schraubenplatzierung und Bewegungsablauf:
- Optimale Fixierung: Die Schraubenlöcher müssen strategisch positioniert werden, um eine stabile Fixierung in Bereichen mit guter Knochenqualität zu erreichen, wobei Frakturlinien oder Bereiche mit Knochenverlust zu vermeiden sind.
- Winkel der Schraube: AM ermöglicht die Gestaltung von Schraubenlöchern mit spezifischen Winkeln, so dass Chirurgen die Schrauben für einen optimalen Halt ausrichten können (z. B. bikortikale Fixierung) oder um darunter liegende Strukturen zu vermeiden. Die Verriegelungsschraubentechnologie, bei der der Schraubenkopf in das Plattenloch eingeschraubt wird, kann ebenfalls zur Verbesserung der Stabilität eingesetzt werden, so dass ein winkelstabiles Konstrukt entsteht. Das Design muss sicherstellen, dass ausreichend Material um das Schraubenloch herum vorhanden ist, um die Verriegelungsmechanismen zu unterstützen.
- Chirurgischer Zugang: Es muss berücksichtigt werden, wie der Chirurg während der Operation Zugang zu den Schraubenlöchern erhält. Die Platzierung und Abwinkelung der Löcher muss einen praktikablen Zugang für Bohrer und Schraubendreher ermöglichen.
4. Einbeziehung von porösen Strukturen und Gitterkonstruktionen:
Dies ist eine einzigartige Fähigkeit der AM, die die Osseointegration verbessern soll.
- Zweck: Durch die Gestaltung einer kontrollierten Porosität in der knochenberührenden Oberfläche oder sogar in Teilen des Plattenkörpers kann das Implantat das Einwachsen von Knochenzellen in seine Struktur fördern, was mit der Zeit zu einer stärkeren biologischen Fixierung führt. Dies kann besonders für die langfristige Stabilität und die Lastübertragung von Vorteil sein.
- Entwurfsparameter:
- Einheit Zellentyp: Verschiedene sich wiederholende geometrische Formen (z. B. kubisch, rautenförmig, gyroid) können zur Erstellung des Gitters verwendet werden. Die Wahl wirkt sich auf die mechanischen Eigenschaften, die Durchlässigkeit und die Druckbarkeit aus.
- Prozentsatz der Porosität: Der gesamte Hohlraum innerhalb des Gitters (z. B. 50-80 %). Eine höhere Porosität begünstigt das Einwachsen, verringert aber die mechanische Festigkeit.
- Strebe/Wanddicke: Der Durchmesser der einzelnen Balken oder Wände, die das Gitter bilden. Er muss für die Druckbarkeit und strukturelle Integrität ausreichen, aber dünn genug sein, um Zellmigration und Vaskularisierung zu ermöglichen. Die Mindestgröße der druckbaren Merkmale des AM-Systems ist eine wichtige Einschränkung.
- Standort: Die Porosität wird in der Regel an der dem Knochen zugewandten Oberfläche oder in bestimmten Regionen angebracht, in denen die biologische Fixierung im Vordergrund steht und die mechanische Belastung geringer ist. Vollständig poröse Platten sind aufgrund der Festigkeitsanforderungen im Allgemeinen nicht machbar.
- Topologie-Optimierung: Hochentwickelte Softwaretools können die Materialverteilung innerhalb des Plattenentwurfs auf der Grundlage der zu erwartenden Belastungen automatisch optimieren, um Gewicht und Volumen zu minimieren und gleichzeitig die erforderliche Festigkeit und Steifigkeit zu erhalten. Dies kann zu sehr organisch aussehenden, effizienten Strukturen führen.
5. Design for Manufacturability (DfAM-Prinzipien):
Die Konstruktion für AM unterscheidet sich von der Konstruktion für die maschinelle Bearbeitung. Zu den wichtigsten Überlegungen gehören:
- Unterstützende Strukturen: Die meisten PBF-Prozesse erfordern Stützstrukturen, um das Teil auf der Bauplatte zu verankern, Verformungen durch thermische Spannungen zu verhindern und überhängende Merkmale während des Bauprozesses zu stützen. Diese Stützen müssen in der Nachbearbeitung entfernt werden. Gute Konstruktionspraxis zielt darauf ab:
- Überhänge minimieren: Ausrichtung des Teils auf der Bauplatte, um den Bedarf an Stützen zu verringern. Selbsttragende Winkel (typischerweise >45 Grad zur Horizontalen bei Titan) sollten nach Möglichkeit verwendet werden.
- Zugängliche Stützen entwerfen: Sicherstellen, dass die Stützen leicht zugänglich sind und entfernt werden können, ohne die Implantatoberfläche zu beschädigen. Die Kontaktpunkte der Stützen sollten idealerweise auf unkritischen Oberflächen liegen.
- Wanddicke und Größe der Merkmale: Es gibt Mindestwandstärken und -größen, die durch das AM-Verfahren vorgegeben sind (Spotgröße des Laser-/Elektronenstrahls, Größe der Pulverpartikel). Die Designs müssen diese Grenzwerte einhalten (z. B. typischerweise >0,3-0,5 mm für Wände). Scharfe Innenecken sollten vermieden werden (Verwendung von Verrundungen), um Spannungskonzentrationen zu verringern.
- Reststress-Management: Ausrichtung und Geometrie der Teile können den Aufbau von Eigenspannungen während der schichtweisen Erwärmungs- und Abkühlungszyklen beeinflussen. Konstruktionsentscheidungen und optimierte Prozessparameter können dazu beitragen, Verzug oder Rissbildung zu vermeiden.
- Entfernung von Puder: Bei Teilen mit inneren Kanälen oder komplexen Gitterstrukturen muss sichergestellt werden, dass nicht verschmolzenes Pulver nach dem Druck vollständig entfernt werden kann. Eingeschlossenes Pulver ist in einem medizinischen Implantat inakzeptabel. Zu den Designüberlegungen gehören Entwässerungslöcher oder ausreichend große Kanäle für eine effektive Reinigung.
6. Zusammenarbeit und Workflow:
- Chirurg-Ingenieur-Partnerschaft: Ein wirksames individuelles Implantatdesign erfordert eine enge Zusammenarbeit zwischen dem Chirurgen (der den klinischen Bedarf und die Anatomie kennt) und dem Biomedizintechniker (der die DfAM-Prinzipien und die Materialwissenschaft kennt). Iterative Designüberprüfungen sind üblich.
- Software-Tools: Es wird eine Reihe von Software verwendet: Software für die medizinische Bildverarbeitung, CAD-Software, Simulationssoftware (FEA – Finite-Elemente-Analyse) zur Vorhersage der mechanischen Leistung unter Belastung und Software für die Vorbereitung von AM-Bauten.
- Regulatorische Erwägungen: Die Konstruktionen müssen den einschlägigen Vorschriften für Medizinprodukte entsprechen (z. B. FDA-Richtlinien, MDR in Europa). Design History Files, Risikobewertungen und Validierungsdokumentation sind wesentliche Bestandteile, die von Medizinprodukteherstellern gefordert und von ihren Großhändlern und Krankenhauskunden erwartet werden.
Die Zusammenarbeit mit einem erfahrenen 3D-Druck-Dienstleister für Metall wie Met3dp, die nicht nur über Fachwissen im Druck, sondern auch in der Designoptimierung für medizinische Anwendungen unter Verwendung von Materialien wie Ti-6Al-4V ELI verfügen, können diesen komplexen Prozess erheblich rationalisieren und sicherstellen, dass das endgültige Implantat die hohen Anforderungen an Passform, Funktion und Sicherheit erfüllt. Ihr Verständnis der Fähigkeiten ihrer spezifischen AM-Systeme (wie das hochpräzise SEBM) fließt von Anfang an in den Designprozess ein.

Erreichen von Präzision: Toleranz, Oberflächengüte und Maßgenauigkeit bei 3D-gedruckten Implantaten
Während die Designfreiheit der additiven Fertigung von Metall ein großer Vorteil ist, müssen die daraus resultierenden Implantate auch strenge Anforderungen an die Präzision erfüllen. Maßgenauigkeit, angemessene Toleranzen und eine kontrollierte Oberflächenbeschaffenheit sind entscheidend für die ordnungsgemäße Funktion, Sicherheit und Biokompatibilität von 3D-gedruckten nicht-tragenden Knochenplatten. Ingenieure, die diese Komponenten entwerfen, Hersteller, die sie produzieren, und Beschaffungsspezialisten, die sie einkaufen, brauchen ein klares Verständnis davon, welche Präzisionsniveaus mit aktuellen AM-Technologien wie SLM und SEBM erreichbar sind, von den Faktoren, die sie beeinflussen, und von der Rolle der Nachbearbeitung.
Maßgenauigkeit und Toleranzen:
Die Maßgenauigkeit gibt an, wie genau das gefertigte Teil mit den im digitalen CAD-Modell angegebenen Nennmaßen übereinstimmt. Die Toleranz definiert den zulässigen Abweichungsbereich für ein bestimmtes Maß.
- Erreichbare Toleranzen (As-Built): Für Ti-6Al-4V ELI-Teile, die mit gut kontrollierten PBF-Prozessen (SLM/SEBM) hergestellt werden, liegen die typischen Maßtoleranzen im eingebauten Zustand oft im Bereich von:
- ± 0,1 mm bis ± 0,3 mm für kleinere Merkmale (< 50 mm)
- ± 0,2% bis ± 0,5% des Nennmaßes für größere Merkmale. Diese Werte sind allgemeine Richtlinien und können je nach Teilegeometrie, Größe, Bauausrichtung, spezifischer Maschinenkalibrierung und verwendeten Prozessparametern erheblich variieren. Unternehmen wie Met3dp streben durch strenge Prozesskontrolle und fortschrittliche Maschinentechnologie wie SEBM ein hohes Maß an Genauigkeit und Wiederholbarkeit an.
- Faktoren, die die Genauigkeit beeinflussen:
- Thermische Effekte: Das schnelle Schmelzen und Erstarren bei PBF-Prozessen führt zu thermischer Ausdehnung und Kontraktion, was innere Spannungen verursacht, die zu leichten Verformungen oder Schrumpfungen führen können. Ein sorgfältiges Wärmemanagement während der Herstellung und eine angemessene Wärmebehandlung danach sind entscheidend.
- Orientierung aufbauen: Die Ausrichtung des Teils auf der Bauplattform wirkt sich auf die thermischen Gradienten, die Anforderungen an die Stützstruktur und den Treppeneffekt bei gekrümmten oder abgewinkelten Oberflächen aus, was alles die endgültige Genauigkeit beeinflusst.
- Laser-/Elektronenstrahl Spotgröße: Der Durchmesser des Energiestrahls begrenzt die Mindestgröße des Merkmals und beeinflusst die Präzision der feinen Details.
- Eigenschaften des Pulvers: Schwankungen in der Größenverteilung oder Morphologie der Pulverpartikel können die Stabilität des Schmelzbades und die Maßhaltigkeit beeinträchtigen.
- Kalibrierung der Maschine: Eine regelmäßige und präzise Kalibrierung des AM-Systems (Scanner, Energiequelle, Z-Achsenbewegung) ist unerlässlich.
- Einhaltung kritischer Toleranzen: Während die Fertigungstoleranzen für die anatomische Gesamtkonformität einer Knochenplatte oft ausreichend sind, können bestimmte Merkmale eine engere Kontrolle erfordern. Zum Beispiel:
- Schraubenlöcher: Die Geometrie des Durchmessers und des Verriegelungselements kann engere Toleranzen erfordern, als sie im Ist-Zustand möglich sind.
- Passende Oberflächen: Schnittstellen, die für die Verbindung mit anderen Komponenten oder chirurgischen Instrumenten vorgesehen sind, erfordern möglicherweise eine höhere Präzision. In solchen Fällen werden in der Regel nach dem Druck sekundäre Bearbeitungsvorgänge durchgeführt.
Oberflächengüte (Rauhigkeit):
Die Oberflächenbeschaffenheit, die in der Regel durch die durchschnittliche Rauheit (Ra) gemessen wird, ist ein weiteres wichtiges Merkmal von 3D-gedruckten Implantaten.
- Oberflächenrauhigkeit im Ist-Zustand: PBF-Prozesse erzeugen naturgemäß Teile mit einer spürbaren Oberflächenrauhigkeit, die auf die schichtweise Beschaffenheit und die teilweise geschmolzenen Pulverpartikel zurückzuführen ist, die an der Oberfläche haften. Typische "as-built" Ra-Werte für Ti-6Al-4V ELI können im Bereich von:
- 5 µm bis 20 µm (Mikrometer) oder noch höher, je nach Ausrichtung der Oberfläche (nach oben, nach unten, vertikale Wände) und Prozessparametern. Nach unten gerichtete Oberflächen, die Abstützungen benötigen, weisen nach dem Entfernen der Abstützung häufig eine höhere Rauheit auf.
- Einfluss der Oberflächenbeschaffenheit:
- Biokompatibilität: Zwar ist die inhärente Biokompatibilität von Titan ausgezeichnet, doch kann eine übermäßige Rauheit die Oberfläche für die Ionenfreisetzung potenziell vergrößern (obwohl sie bei einer stabilen TiO2-Schicht in der Regel minimal ist) und die anfänglichen Zellinteraktionen beeinflussen. Sehr raue Oberflächen können auch Bakterien beherbergen, wenn sie nicht ordnungsgemäß gereinigt und sterilisiert werden, obwohl dies umstritten ist.
- Müdigkeit Leben: Oberflächenrauhigkeit kann als Ausgangspunkt für Ermüdungsrisse dienen. Glattere Oberflächen führen im Allgemeinen zu besseren Ermüdungseigenschaften, was selbst für nicht tragende Platten, die geringen zyklischen Belastungen ausgesetzt sind, wichtig ist.
- Reibung und Abnutzung: Obwohl die Oberflächenbeschaffenheit bei statischen Knochenplatten weniger kritisch ist als bei Gelenkimplantaten, kann sie die Reibung beeinflussen, wenn die Platte mit Weichteilen oder Instrumenten in Kontakt kommt.
- Verbesserung der Oberflächengüte: Die Oberflächen im Rohzustand sind nur selten ohne Modifikation für die endgültige Implantation geeignet. Zur Reduzierung der Rauheit werden verschiedene Nachbearbeitungstechniken eingesetzt:
- Abrasives Strahlen (Perlstrahlen): Üblich zur Erzielung einer gleichmäßigen matten Oberfläche und zur Entfernung lose anhaftender Partikel (Ra typischerweise 3-6 µm).
- Massenveredelung (Taumeln, Gleitschleifen): Verwendung von Schleifmitteln in einer rotierenden oder vibrierenden Trommel zum Glätten von Oberflächen und Abrunden von Kanten (Ra kann 1-3 µm erreichen).
- Elektropolieren: Ein elektrochemisches Verfahren, bei dem eine mikroskopisch kleine Materialschicht abgetragen wird, so dass eine sehr glatte, saubere und korrosionsbeständige Oberfläche entsteht (Ra oft < 0,5 µm).
- Manuelles Polieren: Arbeitsintensiv, kann aber bei Bedarf spiegelglatte Oberflächen auf bestimmten Flächen erzielen.
Qualitätskontrolle und Validierung:
Für die Patientensicherheit und die Einhaltung gesetzlicher Vorschriften ist es von entscheidender Bedeutung, dass jede individuell gedruckte 3D-Knochenplatte die vorgegebenen Abmessungen und Oberflächenbeschaffenheiten erfüllt. Dies beinhaltet:
- Prozess-Validierung: Hersteller von Medizinprodukten müssen ihren gesamten AM-Prozess (einschließlich Design, Druck, Nachbearbeitung, Reinigung, Sterilisation) validieren, um eine konsistente Produktion gemäß den Spezifikationen nachzuweisen (z. B. gemäß den Qualitätsmanagementnormen ISO 13485).
- Prüfung der Abmessungen: Verwendung von Werkzeugen wie Koordinatenmessmaschinen (CMM), 3D-Scannern (strukturiertes Licht oder Laser) und herkömmlichen Messschiebern/Mikrometern zur Überprüfung kritischer Abmessungen anhand des CAD-Modells und der Zeichnungen.
- Messung der Oberflächenrauhigkeit: Einsatz von Profilometern zur Quantifizierung der Oberflächenrauheit (Ra, Rz, usw.) auf kritischen Oberflächen.
- Zerstörungsfreie Prüfung (NDT): Mit Techniken wie Röntgen- oder CT-Scans lassen sich innere Merkmale (z. B. Gitterstrukturen) untersuchen und mögliche Defekte (z. B. Porosität oder eingeschlossenes Pulver) erkennen, ohne das Teil zu beschädigen.
- Rückverfolgbarkeit: Die lückenlose Rückverfolgbarkeit von der ersten Pulvercharge über den Entwurf, den Druck, die Nachbearbeitung und die Endkontrolle ist für Qualitätssysteme für Medizinprodukte unerlässlich. Dies ist eine wichtige Anforderung für Lieferanten, die den medizinischen Großhandelsmarkt bedienen.
Zusammenfassende Tabelle: Aspekte der Präzision
| Parameter | Typischer Ist-Zustand (Ti-6Al-4V ELI PBF) | Beeinflussende Faktoren | Auswirkungen der Nachbearbeitung | Bedeutung für Implantate |
|---|---|---|---|---|
| Abmessungstoleranz | ±0,1-0,3mm / ±0,2-0,5% | Thermische Belastung, Orientierung, Maschine | Bearbeitung für enge Toleranzen | Kritisch für Passform, Schraubenfunktion, Gegenschnittstellen |
| Oberflächenrauhigkeit (Ra) | 5-20 µm+ | Ausrichtung, Träger, Parameter | Strahlen, Trowalisieren, Polieren | Biokompatibilität, Ermüdungslebensdauer, Zellinteraktion, Reinigung |
| Merkmal Auflösung | ~0,1-0,3 mm | Strahlfleckgröße, Pulvergröße | Bearbeitungen für feine Details | Begrenzung der Mindeststrebengröße in Gittern, scharfe Ecken |
| Innere Porosität | <0,1% (mit Optimierung & HIP) | Prozessparameter, Pulverqualität | HIP reduziert/beseitigt Porosität | Entscheidend für mechanische Integrität, Ermüdungsbeständigkeit |
In Blätter exportieren
Während der 3D-Metalldruck eine unglaubliche geometrische Freiheit bietet, erfordert das Erreichen der erforderlichen Präzision für medizinische Implantate wie Knochenplatten eine sorgfältige DfAM, streng kontrollierte Druckprozesse (wie sie von Herstellern mit fortschrittlichen Geräten wie den SEBM-Systemen von Met3dp angeboten werden) und sorgfältige, validierte Nachbearbeitungsschritte. Das Verständnis des Zusammenspiels zwischen Design, Herstellung und Nachbearbeitung ist der Schlüssel zur Herstellung sicherer, effektiver und zuverlässiger patientenspezifischer orthopädischer Lösungen, die sowohl den klinischen Anforderungen als auch den Erwartungen der Behörden entsprechen.
Wesentliche Nachbearbeitungsschritte für Titan-Knochenplatten: Gewährleistung von Sicherheit und Leistung
Die Herstellung einer maßgenauen Titan-Knochenplatte mit komplexen Merkmalen durch additive Fertigung ist nur ein Teil des Weges. Das frisch aus dem Drucker kommende Teil ist noch nicht für die Implantation geeignet. Eine Reihe entscheidender Nachbearbeitungsschritte ist erforderlich, um das rohe gedruckte Bauteil in ein sicheres, biokompatibles und leistungsstarkes medizinisches Gerät zu verwandeln. In diesen Schritten werden Restspannungen beseitigt, Stützstrukturen entfernt, die Oberflächenbeschaffenheit verfeinert, die Sauberkeit sichergestellt und die Qualität überprüft. Die Hersteller von Medizinprodukten, ihre Zulieferer und die Beschaffungsteams der Krankenhäuser müssen sicherstellen, dass diese Prozesse validiert und gemäß den Vorschriften für Medizinprodukte (z. B. ISO 13485, FDA QSR) genauestens kontrolliert werden.
1. Stressabbau / Wärmebehandlung:
- Zweck: Die schnellen Erwärmungs- und Abkühlungszyklen während des PBF-Drucks führen zu erheblichen Eigenspannungen innerhalb des Ti-6Al-4V-ELI-Teils. Diese Spannungen können bei der Entnahme aus der Bauplatte zu Verformungen führen oder, schlimmer noch, zu einem vorzeitigen Versagen im Betrieb. Eine Wärmebehandlung ist unerlässlich, um diese Spannungen abzubauen und das Gefüge des Materials zu homogenisieren.
- Prozess:
- Stressabbau: Wird in der Regel durchgeführt, während das Teil noch auf der Bauplatte befestigt ist (oder unmittelbar nach der Entnahme), in einem Vakuum- oder Schutzgasofen. Die Temperaturen liegen in der Regel im Bereich von 650-800 °C, gefolgt von einer kontrollierten Abkühlung. Auf diese Weise werden die inneren Spannungen reduziert, ohne dass die beim Druck erzielte Mikrostruktur wesentlich verändert wird.
- Heiß-Isostatisches Pressen (HIP): Dies ist ein sehr empfehlenswerter, oft sogar obligatorischer Schritt für kritische Implantate. Die Teile werden gleichzeitig einer hohen Temperatur (z. B. 900-950 °C für Ti-6Al-4V) und einem hohen Inertgasdruck (z. B. 100-200 MPa) ausgesetzt. HIP schließt effektiv die interne Mikroporosität, die nach dem Druck verbleiben könnte, und führt zu einem vollständig dichten Teil (~99,9 %+) mit verbesserter Ermüdungsfestigkeit und mechanischen Eigenschaften. Außerdem werden Spannungen abgebaut und eine gewisse mikrostrukturelle Homogenisierung erreicht.
- Glühen/Lösungsbehandlung & Alterung: Nach dem HIP oder dem Spannungsabbau können weitere Wärmebehandlungen durchgeführt werden, um bestimmte mechanische Eigenschaften (z. B. Festigkeit, Duktilität) zu optimieren, indem die Alpha-Beta-Phasen-Mikrostruktur je nach den Konstruktionsanforderungen verändert wird.
- Wichtigkeit: Wird die Spannung nicht ordnungsgemäß abgebaut, kann dies die Dimensionsstabilität und die mechanische Integrität beeinträchtigen. HIP verbessert die Ermüdungsleistung erheblich, ein kritischer Sicherheitsfaktor.
2. Entfernen des Teils von der Bauplatte:
- Methode: Die gedruckte(n) Knochenplatte(n) werden in der Regel während des AM-Prozesses mit einer Metallplatte verschmolzen. Sie müssen sorgfältig getrennt werden. Zu den gängigen Methoden gehören:
- Draht-Elektroerosion (Wire EDM): Präzise und mit minimaler mechanischer Belastung.
- Bandsägen: Schneller, aber weniger präzise und kann bei unvorsichtiger Durchführung zu mehr Stress führen.
- Bearbeitungen: Fräsen der Trennschicht.
3. Entfernung der Stützstruktur:
- Zweck: Die Stützen sind während des Drucks notwendig, müssen aber danach vollständig entfernt werden.
- Methoden:
- Manuelle Entfernung: Abbrechen oder Abschneiden von Stützen mit Handwerkzeugen (Zangen, Scheren). Arbeitsintensiv und erfordert Geschick, um die Oberfläche des Teils nicht zu beschädigen.
- Spanende Bearbeitung (CNC): Fräsen oder Schleifen von Stützkonstruktionen, insbesondere in zugänglichen Bereichen.
- Drahterodieren: Kann für komplizierte oder schwer zugängliche Halterungen verwendet werden.
- Herausforderungen: Die Entfernung von Halterungen kann schwierig sein, insbesondere bei komplexen Innengeometrien oder Gitterstrukturen. Das DfAM spielt eine entscheidende Rolle bei der Gestaltung von Halterungen, die sowohl effektiv als auch einfach zu entfernen sind. Eine unvollständige Stützentfernung ist inakzeptabel. Verbleibende Stützstellen erfordern oft ein weiteres Verblenden oder Glätten.
Die Entfernung der Stützen kann arbeitsintensiv sein und birgt die Gefahr, die Oberfläche des Teils zu beschädigen, wenn sie nicht sorgfältig durchgeführt wird. Dies unterstreicht die Bedeutung der DfAM-Prinzipien, die darauf abzielen, den Stützbedarf zu minimieren und Stützen für eine einfachere Entfernung zu konstruieren.
- Zweck: Verbesserung der Oberflächenqualität durch Verringerung der Rauheit, Entfernung von Verunreinigungen und Vorbereitung der Oberfläche für optimale Biokompatibilität und Leistung.
- Gemeinsame Techniken (oft nacheinander eingesetzt):
- Strahlen (Perlen-/Kornstrahlen): Mit Druckluft werden Schleifmittel (z. B. Keramikkugeln, Aluminiumoxid) auf die Oberfläche getrieben. Entfernt Pulverreste, glättet die Oberfläche leicht, erzeugt ein gleichmäßiges, mattes Finish und kann geringfügige Abdrücke von Zeugen entfernen. Die Wahl des Mediums ist entscheidend, um Verunreinigungen zu vermeiden.
- Massenveredelung (Taumeln, Gleitschleifen): Die Teile werden in eine Wanne mit Schleifmittel und flüssiger Masse gelegt. Die Wanne rotiert oder vibriert, wodurch das Medium an den Teilen reibt und die Oberflächen glättet und die Kanten entgratet. Effektiv für Chargen von Teilen, aber weniger kontrolliert für spezifische Oberflächenmerkmale.
- Manuelles Entgraten und Blending: Verwendung von Handwerkzeugen oder Elektrowerkzeugen mit Schleifspitzen zum Entfernen von Graten und zum Angleichen von Oberflächen, insbesondere dort, wo Stützen entfernt wurden.
- Elektropolieren: Ein elektrochemisches Verfahren, bei dem Spitzen auf der Oberfläche bevorzugt entfernt werden, was zu einer sehr glatten (niedrigen Ra), sauberen und oft helleren Oberfläche führt. Es kann auch die Korrosionsbeständigkeit durch Verdickung der passiven Oxidschicht verbessern.
- Eloxieren (Typ II): Ein elektrolytisches Verfahren, bei dem eine kontrollierte Oxidschicht auf der Titanoberfläche entsteht. Wird häufig zur farblichen Kennzeichnung von Implantaten verwendet (unterschiedliche Schichtdicken erzeugen unterschiedliche Interferenzfarben), kann aber auch die Verschleißfestigkeit geringfügig verbessern und möglicherweise die Biokompatibilität beeinflussen.
- Passivierung: Eine chemische Behandlung (in der Regel mit Salpeter- oder Zitronensäure), um freies Eisen oder andere Verunreinigungen von der Oberfläche zu entfernen und die Bildung einer robusten, chromoxidreichen Passivschicht zu gewährleisten (häufiger bei Edelstahl, aber manchmal auch bei Titan).
5. Präzisionsbearbeitung (falls erforderlich):
- Zweck: Zur Erzielung engerer Toleranzen oder spezifischer Oberflächengüten bei kritischen Merkmalen, die durch den AM-Prozess im Ist-Zustand oder nachfolgende Nachbearbeitungsschritte nicht erreicht werden können.
- Anwendungen: Verfeinern von Schraubenlochdurchmessern und Gewinden (insbesondere bei Verriegelungsschrauben), Herstellen von sehr ebenen Passflächen, Bearbeiten von spezifischen Funktionsmerkmalen.
- Erwägungen: Erfordert eine sorgfältige Konstruktion der Halterung, um das komplexe AM-Teil zu halten. Die Bearbeitungsflüssigkeiten müssen biokompatibel sein oder vollständig entfernt werden.
6. Reinigung und Inspektion:
- Zweck: Dies ist ein absolut kritischer Schritt, um alle Verunreinigungen aus dem Herstellungsprozess zu entfernen, einschließlich Pulverrückstände, Bearbeitungsflüssigkeiten, Schleifmittel, Poliermittel und Fingerabdrücke. Die Sauberkeit der Implantate ist für die Biokompatibilität und die Vermeidung von unerwünschten Patientenreaktionen oder Infektionen von größter Bedeutung.
- Prozess: In der Regel handelt es sich um einen mehrstufigen Prozess:
- Erste Spülungen: Beseitigung grober Verunreinigungen.
- Reinigung mit Ultraschall: Verwendung von Hochfrequenz-Schallwellen in speziellen Reinigungslösungen (Reinigungsmittel, Lösungsmittel), um Partikel von komplizierten Merkmalen und porösen Strukturen zu lösen. Es können mehrere Bäder mit unterschiedlichen Lösungen verwendet werden.
- Endspülungen: Verwendung von gereinigtem oder deionisiertem Wasser zur Entfernung von Reinigungsmitteln.
- Trocknen: Verwendung von gefilterter Heißluft oder Vakuumöfen.
- Validierung: Reinigungsverfahren müssen streng validiert werden, um ihre Wirksamkeit bei der Entfernung potenzieller Verunreinigungen auf ein akzeptables Niveau nachzuweisen, was häufig durch chemische Analysen oder Zytotoxizitätstests überprüft wird.
- Inspektion: Sichtprüfungen (oft unter Vergrößerung), Maßprüfungen (CMM, Scannen), Messungen der Oberflächenrauheit und möglicherweise NDT (Röntgen/CT) werden durchgeführt, um sicherzustellen, dass das Teil vor der Freigabe alle Spezifikationen erfüllt.
7. Verpackung und Sterilisation:
- Verpackung: Die gereinigten und geprüften Implantate werden in Reinraumumgebungen verpackt, oft doppelt verpackt und mit einer entsprechenden Kennzeichnung versehen, die eine vollständige Rückverfolgbarkeit ermöglicht (Teilenummer, Chargennummer, Material, ggf. Patienten-ID).
- Sterilisation: Verpackte Implantate werden vor dem chirurgischen Einsatz sterilisiert. Zu den gängigen Methoden für Titanimplantate gehören:
- Autoklavieren (Dampfsterilisation): Häufigste Methode.
- Gamma-Bestrahlung: Wirksam, kann aber manchmal leichte Verfärbungen verursachen.
- Ethylenoxid (EtO) Gas: Bei Metallen ist dies aufgrund möglicher Rückstände weniger üblich. Die gewählte Sterilisationsmethode muss validiert werden, um sicherzustellen, dass sie das erforderliche Sterilitätssicherungsniveau (SAL) erreicht, ohne die Eigenschaften des Implantats zu beeinträchtigen.
Die Bedeutung von integriertem Fachwissen:
Die erfolgreiche Bewältigung dieser komplizierten Nachbearbeitungsschritte erfordert ein hohes Maß an Fachwissen und Spezialausrüstung. Die Zusammenarbeit mit einem vertikal integrierten Partner oder einem hochqualifizierten Lieferantennetz ist entscheidend. Unternehmen wie Met3dpdas Unternehmen bietet Lösungen an, die von der Pulverproduktion bis zu fortschrittlichen Drucksystemen und Anwendungsunterstützung reichen. Es versteht die gesamte Wertschöpfungskette und das kritische Zusammenspiel zwischen Druck und Nachbearbeitung, das für die Herstellung hochwertiger, implantierbarer medizinischer Produkte erforderlich ist. Ihr Fokus auf Prozesskontrolle und Materialqualität bildet eine solide Grundlage für die anschließende Weiterverarbeitung. Beschaffungsteams, die potenzielle Lieferanten bewerten, müssen deren Nachbearbeitungsfähigkeiten und Qualitätsmanagementsysteme ebenso streng prüfen wie ihre Drucktechnologie.

Herausforderungen beim 3D-Druck von Knochenplatten meistern: Lösungen und bewährte Praktiken für Zulieferer
Die additive Fertigung von Metall bietet zwar ein transformatives Potenzial für individuelle Knochenplatten, doch der Weg vom digitalen Design zum sterilen Implantat ist nicht ohne Herausforderungen. Die erfolgreiche Implementierung dieser Technologie erfordert die Vorwegnahme potenzieller Probleme und die Einführung robuster Prozesse und bewährter Verfahren, um diese zu entschärfen. Für Hersteller von Medizinprodukten, Großhandelslieferanten und ihre Produktionspartner ist das Verständnis und die Bewältigung dieser Herausforderungen der Schlüssel zur Gewährleistung einer gleichbleibenden Qualität, der Einhaltung gesetzlicher Vorschriften und letztlich der Patientensicherheit.
1. Eigenspannung, Verformung und Rissbildung:
- Herausforderung: Die schnelle, örtlich begrenzte Erwärmung und Abkühlung, die den PBF-Prozessen eigen ist, erzeugt erhebliche thermische Gradienten und daraus resultierende Eigenspannungen innerhalb des Ti-6Al-4V ELI-Teils. Diese Spannungen können dazu führen, dass sich Teile verziehen oder verzerren, insbesondere nach der Entnahme aus der Bauplatte, oder sogar zu Rissen während der Herstellung oder der anschließenden Handhabung führen.
- Lösungen und bewährte Praktiken:
- Optimierte Build-Strategie: Eine sorgfältige Auswahl der Bauausrichtung, die strategische Platzierung von Stützstrukturen (die als Wärmesenken und Verankerungen fungieren) und optimierte Scan-Strategien (z. B. Insel-Scanning, spezifische Schraffurmuster) können die Stressakkumulation minimieren.
- Optimierung der Prozessparameter: Die Feinabstimmung von Parametern wie Laser-/Strahlleistung, Scangeschwindigkeit, Schichtdicke und Kammerheizung (besonders wichtig beim SEBM) ist entscheidend für ein stabiles Schmelzen und einen geringeren Temperaturschock.
- Obligatorische Wärmebehandlung: Die Durchführung von validierten Spannungsabbauzyklen (häufig auf der Bauplatte) und/oder HIP ist unverzichtbar, um Eigenspannungen zu verringern und Verformungen und Risse in Titanbauteilen zu verhindern.
- DfAM: Die Konstruktion von Teilen mit Merkmalen, die die Spannungskonzentration minimieren (z. B. Verrundungen anstelle von scharfen Ecken), und die Vermeidung von großen, abrupten Querschnittsänderungen können helfen.
2. Porosität und Fehlen von Fusionsdefekten:
- Herausforderung: Ein unvollständiges Aufschmelzen der Pulverpartikel oder Instabilitäten im Schmelzbad können zu Hohlräumen (Porosität) im fertigen Teil führen. Diese Defekte können die mechanischen Eigenschaften, insbesondere die Ermüdungsfestigkeit, erheblich beeinträchtigen und als Rissauslöser wirken. Porosität kann durch falsche Prozessparameter, schlechte Pulverqualität oder Gaseinschlüsse entstehen.
- Lösungen und bewährte Praktiken:
- Strenge Parameterentwicklung: Ausführliche Tests (Entwicklung von Parametern “Coupons”), um die optimalen Einstellungen für die Dichte (>99,5% typischerweise erforderlich vor HIP) für die spezifische Maschine, Materialcharge und Geometrietyp zu ermitteln.
- Hochwertiges Pulver: Die Verwendung von Pulver mit kontrollierter Sphärizität, PSD, Fließfähigkeit und geringem internen Gasgehalt ist entscheidend. Die Beschaffung von Pulver von renommierten Anbietern wie Met3dp, die eine fortschrittliche Zerstäubung (GA, PREP) und eine strenge Qualitätskontrolle anwenden, ist von entscheidender Bedeutung. Regelmäßige Pulvertests und sorgfältige Handhabungs-/Recyclingprotokolle sind erforderlich, um eine Zersetzung zu verhindern.
- Prozessüberwachung: Fortgeschrittene AM-Systeme können In-situ-Überwachungsinstrumente (z. B. Schmelzbadüberwachung) enthalten, um Anomalien während der Herstellung zu erkennen, die ein mögliches Eingreifen oder den Ausschuss von Teilen ermöglichen.
- Heiß-Isostatisches Pressen (HIP): Wie bereits erwähnt, ist HIP äußerst wirksam beim Schließen der internen Gasporosität, was die Integrität der Teile erheblich verbessert.
- NDT-Inspektion: Einsatz von Röntgen- oder Mikro-CT-Scans zur Erkennung und Quantifizierung der inneren Porosität in Fertigteilen, insbesondere bei kritischen Anwendungen.
3. Schwierigkeiten bei der Entfernung von Stützstrukturen:
- Herausforderung: Stützen sind zwar notwendig, können aber schwierig und zeitaufwändig zu entfernen sein, insbesondere bei komplexen inneren Kanälen oder empfindlichen Gitterstrukturen. Eine unvollständige Entfernung oder Beschädigung der Teileoberfläche während der Entfernung ist ein Risiko.
- Lösungen und bewährte Praktiken:
- DfAM-Optimierung: Konstruktion von Teilen und Wahl der Bauausrichtung, um den Bedarf an Stützen zu minimieren (Verwendung selbsttragender Winkel) und um sicherzustellen, dass die erforderlichen Stützen zugänglich und so gestaltet sind, dass sie sich leichter lösen lassen (z. B. mit kleineren Kontaktpunkten).
- Spezialisierte Werkzeuge & Techniken: Einsatz geeigneter Werkzeuge (manuell, CNC, Drahterodieren) und Entwicklung spezifischer Verfahren zur Entfernung von Halterungen auf der Grundlage der Geometrie.
- Qualifizierte Techniker: Beschäftigung von gut ausgebildeten Technikern mit Erfahrung im Umgang mit empfindlichen AM-Teilen.
- Oberflächenveredelung: Nach dem Ausbau sind Nachbearbeitungsschritte (Strahlen, Mischen) erforderlich, um die Bereiche zu glätten, an denen Stützen angebracht waren.
4. Pulverkontamination und Management:
- Herausforderung: Metallpulver, insbesondere reaktive Pulver wie Titan, können durch die Einwirkung von Sauerstoff/Feuchtigkeit, durch Kreuzkontamination mit anderen Metallen oder durch unsachgemäße Handhabung verunreinigt werden. Verunreinigtes Pulver kann zu Defekten führen und die Materialeigenschaften beeinträchtigen. Die vollständige Entfernung von ungeschmolzenem Pulver aus komplexen Geometrien ist ebenfalls entscheidend.
- Lösungen und bewährte Praktiken:
- Strenge Protokolle für die Handhabung von Pulver: Lagerung des Pulvers in kontrollierten, inerten Umgebungen; Verwendung spezieller Geräte für bestimmte Materialien; Anwendung strenger Sieb- und Prüfverfahren für neues und recyceltes Pulver.
- Rückverfolgbarkeit: Rückverfolgbarkeit der Chargen für alle verwendeten Pulver.
- Design für Pulverentfernung: Einbau von Entwässerungslöchern oder -kanälen in Konstruktionen mit inneren Hohlräumen oder Gittern zur Erleichterung der Pulverentfernung durch Vibration, Druckluft usw.
- Validierung der Reinigung: Validierung von Reinigungsprozessen, um sicherzustellen, dass alle losen Pulverpartikel entfernt werden. CT-Scans können manchmal verwendet werden, um die Entfernung von Pulver aus internen Kanälen zu überprüfen.
5. Sicherstellung von Biokompatibilität und Sauberkeit:
- Herausforderung: Abgesehen von der inhärenten Biokompatibilität von Ti-6Al-4V ELI darf der gesamte Herstellungsprozess (Druck, Handhabung, Bearbeitung, Endbearbeitung, Reinigung) keine Verunreinigungen oder Oberflächenbedingungen einführen, die eine negative biologische Reaktion hervorrufen könnten. Rückstände von Fertigungsmaterialien (Pulver, Schneidflüssigkeiten, Poliermittel) sind inakzeptabel.
- Lösungen und bewährte Praktiken:
- Materialzertifizierung: Es wird nur medizinisch zertifiziertes Ti-6Al-4V ELI-Pulver von qualifizierten Anbietern verwendet.
- Validierte Nachbearbeitung: Alle Nachbearbeitungsschritte, insbesondere die Reinigung, müssen mit Hilfe analytischer Verfahren (z. B. TOC-Analyse, Zytotoxizitätstests) streng validiert werden, um die Entfernung potenzieller Rückstände auf ein sicheres Niveau nachzuweisen.
- Reinraumumgebung: Durchführung der Endreinigung und Verpackung in einer zertifizierten Reinraumumgebung.
- Biokompatibilitätstests: Durchführung von Biokompatibilitätstests (gemäß ISO 10993) an endgültigen, sterilisierten Teilen als Teil der allgemeinen Produktvalidierung.
6. Konsistente Qualität und Einhaltung gesetzlicher Vorschriften erreichen:
- Herausforderung: Die konsistente Herstellung hochwertiger, patientenspezifischer Implantate, die alle Spezifikationen und behördlichen Anforderungen erfüllen, erfordert ein robustes Qualitätsmanagementsystem (QMS). Schwankungen bei Maschinen, Materialien oder Prozessen können sich auf die Ergebnisse auswirken.
- Lösungen und bewährte Praktiken:
- ISO 13485-Zertifizierung: Die Einführung und Aufrechterhaltung eines nach ISO 13485 (Medizinprodukte - Qualitätsmanagementsysteme) zertifizierten QMS ist für Hersteller von Medizinprodukten und wichtige Lieferanten von grundlegender Bedeutung.
- Prozessvalidierung (IQ/OQ/PQ): Gründliche Validierung aller Geräte (Installationsqualifizierung), Prozesse (Betriebsqualifizierung) und Gewährleistung einer gleichbleibenden Produktqualität (Leistungsqualifizierung).
- Standardarbeitsanweisungen (SOPs): Dokumentation und strikte Einhaltung von SOPs für jeden Schritt des Arbeitsablaufs.
- Bedienerschulung: Sicherstellen, dass alle beteiligten Mitarbeiter angemessen geschult sind.
- Kontrolle ändern: Umsetzung strenger Änderungskontrollverfahren für alle Änderungen an Materialien, Prozessen oder Ausrüstung.
- Umfassende Dokumentation: Führen detaillierter Aufzeichnungen (Design History File, Device Master Record, Device History Record) für Rückverfolgbarkeit und Zulassungsanträge.
Um diese Herausforderungen zu meistern, bedarf es einer Kombination aus technologischem Fachwissen, sorgfältiger Prozesskontrolle und einem ausgeprägten Engagement für Qualität. Die Zusammenarbeit mit sachkundigen und erfahrenen Unternehmen wie Met3dp, die nicht nur Ausrüstung und Materialien, sondern auch umfassende Anwendungsunterstützung bieten und die Feinheiten des gesamten additiven Fertigungsablaufs für Medizinprodukte kennen, kann das Risiko für die Hersteller erheblich verringern und sicherstellen, dass Großhändler und Gesundheitsdienstleister sichere, effektive und zuverlässige individuelle Knochenplatten erhalten.
Die Wahl des richtigen 3D-Druckdienstleisters für medizinische Geräte aus Metall: Ein Leitfaden für die Beschaffung
Die Auswahl des richtigen Fertigungspartners ist wohl eine der wichtigsten Entscheidungen bei der Beschaffung von kundenspezifischen 3D-gedruckten nicht-tragenden Knochenplatten. Die einzigartigen Anforderungen an die Herstellung von Medizinprodukten, insbesondere von Implantaten, bedeuten, dass nicht jedes beliebige Unternehmen für die additive Fertigung ausreichend ist. Beschaffungsmanager, Ingenieure und Lieferkettenspezialisten in Medizintechnikunternehmen, Großhändlern und Krankenhausnetzwerken benötigen einen strengen Bewertungsprozess, um Lieferanten zu identifizieren, die in der Lage sind, durchgängig sichere, effektive und konforme Produkte zu liefern. Die Wahl des falschen Partners kann zu Verzögerungen, Qualitätsproblemen und der Nichteinhaltung von Vorschriften führen und möglicherweise die Patientensicherheit gefährden.
Im Folgenden sind die wichtigsten Kriterien aufgeführt, die bei der Bewertung potenzieller Anbieter von Metall-AM-Dienstleistungen für Medizinproduktkomponenten wie Ti-6Al-4V ELI-Knochenplatten zu berücksichtigen sind:
1. Zertifizierungen und Einhaltung gesetzlicher Vorschriften:
- ISO 13485:2016: Dies ist die nicht verhandelbar internationaler Standard für Qualitätsmanagementsysteme (QMS) für Hersteller und Lieferanten von Medizinprodukten. Die Zertifizierung beweist, dass der Anbieter ein QMS eingerichtet hat und unterhält, das in der Lage ist, die Anforderungen der Kunden und der geltenden Vorschriften durchgängig zu erfüllen. Eine fehlende Zertifizierung sollte ein sofortiges Ausschlusskriterium für die Implantatherstellung sein.
- FDA-Registrierung / Konformität (falls zutreffend): Wenn das fertige Medizinprodukt in den Vereinigten Staaten vermarktet werden soll, muss der Fertigungspartner möglicherweise bei der FDA registriert sein und deren Qualitätssystemverordnung (QSR) gemäß 21 CFR Teil 820 einhalten. Die Erfahrung des Lieferanten mit den Anforderungen der FDA ist von entscheidender Bedeutung.
- Andere Zertifizierungen (z. B. ISO 9001): Während die ISO 13485 im Vordergrund steht, kann die ISO 9001 (allgemeines QMS) auf eine umfassendere Verpflichtung zu Qualitätsgrundsätzen hinweisen.
2. Nachgewiesene Erfahrung mit medizinischen Geräten:
- Erfolgsbilanz: Suchen Sie nach Anbietern mit nachweislicher Erfahrung speziell in der Herstellung von orthopädischen Implantaten, vorzugsweise Titankomponenten, die mittels AM hergestellt werden. Fragen Sie nach Fallstudien, Referenzen (unter Wahrung der Vertraulichkeit) und Belegen für erfolgreich hergestellte Teile, die den klinischen Anforderungen entsprechen.
- Verständnis für den medizinischen Bedarf: Der Anbieter sollte die einzigartigen Herausforderungen und die kritische Natur medizinischer Implantate verstehen, einschließlich der Anforderungen an die Biokompatibilität, die Sterilisationskompatibilität und die Bedeutung der anatomischen Genauigkeit. Er sollte die Sprache der Medizinprodukteentwicklung sprechen.
3. Materialkenntnis und Rückverfolgbarkeit:
- Materialbeschaffung: Woher beziehen sie ihr Ti-6Al-4V ELI-Pulver? Stammt es von seriösen Lieferanten, die die medizinischen Normen (z. B. ASTM F136) einhalten? Führen sie eine Materialeingangskontrolle und -prüfung durch?
- Pulverbehandlung & Management: Gibt es strenge Protokolle für die Lagerung, die Handhabung, das Sieben, das Recycling und das Testen des Pulvers, um Verunreinigungen zu vermeiden und die Konsistenz zu gewährleisten? Die Kreuzkontamination zwischen verschiedenen Metallarten ist ein großes Risiko, das kontrolliert werden muss.
- Vollständige Rückverfolgbarkeit der Materialien: Der Anbieter muss eine durchgängige Rückverfolgbarkeit nachweisen, die das endgültige Implantat mit der verwendeten Pulvercharge, den Verarbeitungsparametern, den Bedienerprotokollen und den Qualitätskontrollen in Verbindung bringt. Dies ist wichtig für die Untersuchung, falls Probleme auftreten sollten.
4. Technologische Fähigkeiten und Prozesskontrolle:
- AM-Technologie: Setzen sie eine geeignete, gut gewartete AM-Technologie ein (z. B. SLM, SEBM)? Die verschiedenen Technologien haben unterschiedliche Nuancen. Zum Beispiel die SEBM-Technologie, wie sie von Met3dpbei der Herstellung von Bauteilen aus Titan werden oft höhere Temperaturen verwendet, was die Eigenspannung in den Bauteilen verringern und die Nachbearbeitung vereinfachen kann. Verstehen Sie deren Maschinenpark, Wartungspläne und Kalibrierungsverfahren.
- Prozess-Validierung: Haben sie ihre Druckverfahren (IQ/OQ/PQ) speziell für medizinische Anwendungen aus Ti-6Al-4V ELI validiert? Dies schafft Vertrauen in die Konsistenz und Reproduzierbarkeit ihrer Produktionsergebnisse.
- Steuerung der Parameter: Weisen sie eine robuste Kontrolle über kritische Prozessparameter auf (Laser-/Strahlleistung, Scangeschwindigkeit, Schichtdicke, Atmosphärensteuerung, Temperatur)?
5. Umfassende Nachbearbeitungsmöglichkeiten:
- In-House vs. Outsourced: Führt der Anbieter kritische Nachbearbeitungsschritte (Wärmebehandlung/HIP, Entfernen von Stützen, Bearbeitung, Endbearbeitung, Reinigung) intern durch oder verlässt er sich auf qualifizierte externe Partner? Falls ausgelagert, wie werden diese Anbieter verwaltet und qualifiziert?
- Validierte Prozesse: Entscheidend ist, ob diese Nachbearbeitungsschritte, insbesondere die Reinigung und die Wärmebehandlung/HIP, ordnungsgemäß validiert sind, um die Normen für Medizinprodukte zu erfüllen Verlangen Sie Nachweise über Validierungsprotokolle und -berichte. Die HIP-Fähigkeit ist besonders wichtig, um eine maximale Dichte und Ermüdungsleistung bei kritischen Implantaten zu gewährleisten.
- Optionen für die Endbearbeitung: Können sie die geforderte Oberflächengüte durch kontrollierte Verfahren wie Perlstrahlen, Trommeln oder Elektropolieren erreichen?
6. Ein solides Qualitätsmanagementsystem (QMS):
- Jenseits der Zertifizierung: Schauen Sie genauer hin als nur auf das ISO 13485-Zertifikat. Bewerten Sie den Reifegrad und die Umsetzung des QMS. Dazu gehören die Kontrolle der Dokumentation, das Führen von Aufzeichnungen, Risikomanagementpraktiken, Verfahren für Korrektur- und Vorbeugungsmaßnahmen (CAPA), interne Audits und Managementprüfungsverfahren.
- Inspektion und Prüfung: Welche Möglichkeiten bieten sie für die Dimensionsprüfung (CMM, 3D-Scannen), die Oberflächenanalyse, die Materialprüfung und möglicherweise die zerstörungsfreie Prüfung (Röntgen/CT)? Wie werden die Prüfergebnisse dokumentiert und mit der Rückverfolgbarkeit der Teile verknüpft?
7. Unterstützung bei Design und Technik:
- DfAM-Fachwissen: Bietet der Anbieter Unterstützung beim Design for Additive Manufacturing? Kann er effektiv mit Ihrem chirurgischen oder technischen Team zusammenarbeiten, um die Designs hinsichtlich Druckbarkeit, Funktionalität und Kosteneffizienz zu optimieren und gleichzeitig die klinischen Anforderungen zu erfüllen?
- Software und Arbeitsablauf: Welche Software-Tools verwenden sie? Wie werden Entwurfsiterationen verwaltet und die Versionskontrolle für patientenspezifische Dateien sichergestellt?
8. Kapazität, Vorlaufzeit und Skalierbarkeit:
- Durchlaufzeit: Können sie die für die klinische Planung oder die Anforderungen der Lieferkette erforderlichen Vorlaufzeiten durchgängig einhalten? Informieren Sie sich über die typischen Vorlaufzeiten für kundenspezifische Ti-6Al-4V ELI-Platten und über das Verfahren zur Bearbeitung von Eilaufträgen (falls erforderlich und zu welchen Kosten).
- Produktionskapazität: Verfügen sie über ausreichende Maschinenkapazitäten und Personal, um Ihren aktuellen und potenziellen künftigen Mengenbedarf zu decken, was insbesondere für Großhändler relevant ist, die größere Chargenaufträge oder laufende Lieferverträge planen?
- Skalierbarkeit: Können sie die Produktion je nach Nachfrageschwankungen erhöhen oder verringern?
9. Kommunikation, Transparenz und Partnerschaft:
- Reaktionsfähigkeit: Reagieren sie auf Anfragen und Angebotsanfragen (RFQ)? Ist die Kommunikation klar und professionell?
- Transparenz: Sind sie offen für ihre Prozesse, Fähigkeiten und Grenzen? Ist ihre Preisstruktur klar und verständlich?
- Kollaborativer Ansatz: Betrachten sie die Beziehung als eine echte Partnerschaft, die auf den gegenseitigen Erfolg und die Patientensicherheit ausgerichtet ist, und nicht nur als eine transaktionale Beziehung?
10. Vertraulichkeit und Schutz des geistigen Eigentums (IP):
- Datensicherheit: Wie gewährleisten sie die Vertraulichkeit und Sicherheit sensibler Patientendaten (Scandaten) und geschützter Implantatdesigns? Geheimhaltungsvereinbarungen (NDAs) sind gängige Praxis.
Lieferanten-Audit:
Bei der Herstellung kritischer Implantate ist ein gründliches Lieferantenaudit sehr zu empfehlen. Dazu gehören ein Besuch vor Ort (oder die Durchführung eines detaillierten Fernaudits), die Überprüfung der Unterlagen, die Befragung wichtiger Mitarbeiter und die Beobachtung der Prozesse aus erster Hand, um die Fähigkeiten und die Einhaltung dieser Kriterien zu überprüfen.
Die Wahl des richtigen Metall-AM-Dienstleisters ist eine strategische Entscheidung. Sie erfordert eine sorgfältige Due-Diligence-Prüfung, die über den bloßen Vergleich von Preisangeboten hinausgeht. Die Konzentration auf Qualität, Compliance, Fachwissen und einen kooperativen Ansatz führt zu einer zuverlässigen Versorgung mit leistungsstarken, patientenspezifischen Knochenplatten, die den anspruchsvollen Standards der medizinischen Industrie entsprechen.
Verständnis der Kostenfaktoren und Vorlaufzeiten für individuelle Knochenplatten: Einblicke in Großhandel und Produktion
Eine der wichtigsten Überlegungen für Medizintechnikunternehmen, Beschaffungsabteilungen von Krankenhäusern und Großhändlern, die sich für individuell gedruckte 3D-Knochenplatten interessieren, sind die damit verbundenen Kosten und Produktionsfristen. Die patientenindividuelle Herstellung bietet zwar erhebliche klinische Vorteile, unterscheidet sich aber von der Wirtschaftlichkeit der in Massenproduktion hergestellten Standardimplantate. Ein klares Verständnis der Kostentreiber und Vorlaufzeitkomponenten ist für eine genaue Budgetierung, Operationsplanung und ein effektives Lieferkettenmanagement unerlässlich.
Kostenfaktoren für individuell gedruckte 3D-Knochenplatten:
Der Endpreis einer maßgefertigten Ti-6Al-4V ELI-Knochenplatte wird durch ein komplexes Zusammenspiel von Faktoren beeinflusst. Es handelt sich selten um einen einfachen Stückpreis wie bei Standardprodukten. Zu den wichtigsten Faktoren gehören:
- Entwurfskomplexität und Entwicklungszeit:
- Patientenspezifisches Design: Die Erstellung eines individuellen Designs für jeden Patienten erfordert viel Zeit für die Bildsegmentierung, CAD-Modellierung, mögliche FEA-Simulationen und Designbesprechungen mit dem Chirurgen. Dieser Aufwand im Vorfeld trägt erheblich zu den Kosten einer einzelnen individuellen Platte bei.
- Geometrische Komplexität: Komplizierte Merkmale wie komplexe Krümmungen, dünne Wände, integrierte Gitterstrukturen oder zahlreiche abgewinkelte Schraubenlöcher erhöhen die Konstruktionszeit und möglicherweise auch die Druck-/Nachbearbeitungsschwierigkeiten und wirken sich auf die Kosten aus.
- Größe und Volumen des Teils:
- Materialverbrauch: Größere oder dickere Platten erfordern mehr Ti-6Al-4V ELI-Pulver, ein relativ teures Rohmaterial.
- Build Volume Occupation: Der Platz, den das Teil in der Baukammer der AM-Maschine einnimmt (seine Bounding Box), beeinflusst, wie viele Teile gleichzeitig gedruckt werden können, was sich auf die Maschinennutzungskosten auswirkt. Größere Teile beanspruchen mehr Platz und erfordern möglicherweise längere Bauzeiten.
- Additive Fertigung (Druck) Zeit:
- Maschine Stundensatz: AM-Maschinen stellen eine erhebliche Kapitalinvestition dar und verursachen Betriebskosten (Energie, Inertgas, Wartung). Die Preisgestaltung ist oft mit der Zeit verbunden, die für den Druck des Teils/der Teile benötigt wird. Diese hängt von der Höhe des Teils (Anzahl der Schichten), dem Volumen und den gewählten Prozessparametern (z. B. Schichtdicke, Scangeschwindigkeit) ab.
- Nesting-Effizienz: Bei der Serienproduktion (relevant für Händler, die Semi-Custom-Serien oder Forschungschargen auf Lager haben) hat die Frage, wie effizient mehrere Teile in einem einzigen Build verschachtelt werden können, einen erheblichen Einfluss auf die Druckkosten pro Teil.
- Nachbearbeitungsintensität:
- Wärmebehandlung / HIP: Der Spannungsabbau und insbesondere das heißisostatische Pressen (HIP) erfordern spezielle Ofenanlagen und verursachen einen erheblichen Zeit- und Kostenaufwand, sind aber für die Qualität entscheidend.
- Unterstützung bei der Entfernung: Komplexe Teile mit umfangreichen oder schwer zugänglichen Halterungen erfordern mehr manuelle Arbeit oder spezielle Techniken (z. B. EDM), was die Kosten erhöht.
- Oberflächenveredelung: Der Grad der erforderlichen Oberflächenbearbeitung (z. B. einfaches Perlstrahlen gegenüber Elektropolieren oder manuellem Polieren) wirkt sich direkt auf den Arbeitsaufwand und die Bearbeitungszeit aus.
- Bearbeitungen: Jede erforderliche CNC-Bearbeitung für kritische Merkmale erhöht die Rüstzeit, die Maschinenzeit und die Arbeitskosten.
- Reinigung und Inspektion: Strenge, validierte Reinigungsverfahren und gründliche Qualitätskontrollen (visuell, dimensional, NDT) tragen zu den Gesamtkosten bei, sind aber für Medizinprodukte unerlässlich.
- Materialkosten:
- Ti-6Al-4V ELI-Pulver: Titanpulver in medizinischer Qualität, das strenge Normen (z. B. ASTM F136) erfüllt, ist aufgrund der Rohstoffe und der speziellen Produktionsverfahren (wie PREP oder hochreines GA) mit hohen Kosten verbunden. Die Qualität des Pulvers wirkt sich direkt auf die Qualität des Endprodukts aus.
- Qualitätssicherung und behördliche Gemeinkosten:
- QMS-Wartung: Der Betrieb eines nach ISO 13485 zertifizierten QMS ist mit laufenden Kosten für Dokumentation, Audits, Schulungen und Konformitätsaktivitäten verbunden, die in den Preis einkalkuliert sind.
- Validierungsanstrengungen: Die Validierung von Prozessen erfordert erhebliche Vorabinvestitionen.
- Chargengröße und Auftragsvolumen:
- Einzelner Patient vs. Batch: Die Herstellung einer einzigen, patientenindividuellen Platte verursacht höhere Stückkosten, da mehr Zeit für das Design und die Einrichtung der Maschine benötigt wird.
- Mengenrabatte: Großhändler oder Krankenhäuser, die größere Mengen standardisierter (oder halbindividueller) Designs bestellen, können durch effizientere Verschachtelung, optimierte Arbeitsabläufe und amortisierte Einrichtungskosten Größenvorteile erzielen, was zu niedrigeren Preisen pro Teil führt. Sprechen Sie mit potenziellen Lieferanten über Preisstaffeln für große Mengen.
- Expedit-Gebühren:
- Dringende Aufträge: Bei der Beantragung von Lieferzeiten, die über den Standard hinausgehen, fallen häufig Eilgebühren an, um Überstunden, die Neupriorisierung von Maschinenplänen und eine eventuelle Sonderbearbeitung abzudecken.
Komponenten der Vorlaufzeit und typische Dauer:
Unter Vorlaufzeit versteht man die Gesamtzeit, die von der Auftragserteilung (oder der Fertigstellung des Designs) bis zum Versand des fertigen, geprüften Implantats vergeht. Sie ist entscheidend für die chirurgische Terminplanung und die Bestandsplanung.
- Schlüsselphasen, die zur Vorlaufzeit beitragen:
- Entwurfsphase (falls zutreffend): Segmentierung, CAD-Design, Überprüfungs-/Genehmigungszyklen (kann je nach Komplexität und Reaktionsfähigkeit Stunden bis Tage dauern).
- Auftragsabwicklung & Terminplanung: Einstellen des Auftrags in die Produktionswarteschlange (kann je nach Arbeitsbelastung des Lieferanten von sofort bis zu mehreren Tagen dauern).
- Build Setup & Drucken: Vorbereitung der Druckdatei, Einrichten der Maschine und die eigentliche AM-Druckzeit (in der Regel 12 Stunden bis mehrere Tage, je nach Bauhöhe und Teiledichte).
- Abkühlung und Entfettung: Die Baukammer abkühlen lassen, die Bauplatte entfernen und das nicht geschmolzene Pulver vorsichtig entfernen (mehrere Stunden).
- Wärmebehandlung / HIP: Zykluszeit des Ofens, einschließlich Erhitzen, Einweichen, Abkühlen (kann 1-2 Tage dauern, einschließlich Einrichtung).
- Ausbau von Teilen & Ausbau von Stützen: Trennen der Teile von der Bauplatte und Entfernen der Stützen (Stunden bis Tage, je nach Komplexität und Methode).
- Bearbeitung & Oberflächenveredelung: Zeitaufwand für die erforderlichen Bearbeitungen und Oberflächenbehandlungen (Stunden bis Tage).
- Reinigung & Trocknen: Validierte Reinigungszyklen (mehrere Stunden).
- Qualitätsinspektion: Maßkontrollen, Oberflächenprüfung, Überprüfung der Dokumentation (Stunden bis zu einem Tag).
- Verpackung & Versand: Endgültige Vorbereitung für den Versand.
- Typische Gesamtvorlaufzeiten:
- Hochgradig variabel: Es ist schwierig, eine einzige definitive Antwort zu geben, da die Durchlaufzeiten stark von den oben genannten Faktoren abhängen (Komplexität, Chargengröße, Arbeitsbelastung der Lieferanten, erforderliche Nachbearbeitung).
- Allgemeiner Bereich (Custom Plate): Für eine einzelne patientenspezifische Ti-6Al-4V ELI-Knochenplatte, die eine Standard-Nachbearbeitung einschließlich HIP erfordert, liegen die Vorlaufzeiten oft zwischen 1 bis 4 Wochen nach der endgültigen Genehmigung des Entwurfs.
- Eiliger Dienst: Kann die Vorlaufzeiten erheblich verkürzen, ist aber mit hohen Kosten verbunden.
- Batch-Produktion: Die Durchlaufzeiten für größere Chargen können insgesamt länger sein, führen aber zu einer niedrigeren durchschnittlichen Durchlaufzeit pro Teil, sobald die Produktion rationalisiert ist.
Erwartungen managen:
Eine klare Kommunikation zwischen dem Medizintechnikunternehmen/Krankenhaus und dem AM-Dienstleister ist unerlässlich. Das Verständnis der Kostenaufschlüsselung und der Faktoren, die die Vorlaufzeit beeinflussen, ermöglicht eine bessere Planung. Bei der Einholung von Angeboten (RFQs) sollten Sie detaillierte Informationen bereitstellen (CAD-Modelle, wenn möglich, Materialspezifikationen, erforderliche Toleranzen, Oberflächenbeschaffenheit, Losgröße, gewünschter Zeitrahmen), um genaue Preis- und Vorlaufzeitschätzungen zu erhalten. Berücksichtigen Sie beim Vergleich von kundenspezifischen AM-Platten mit herkömmlichen Optionen die Gesamtbetriebskosten, d. h. die potenziellen Einsparungen durch verkürzte OP-Zeiten oder verbesserte Ergebnisse, und nicht nur die Implantatkosten pro Teil.

Häufig gestellte Fragen (FAQ) zu 3D-gedruckten Titan-Knochenplatten
Da sich die additive Fertigung von Metallen in der Orthopädie immer mehr durchsetzt, haben Chirurgen, Beschaffungsspezialisten, Ingenieure und sogar Patienten häufig Fragen zu individuell gedruckten 3D-Knochenplatten aus Titan. Hier finden Sie Antworten auf einige häufig gestellte Fragen:
1. Ist 3D-gedrucktes Ti-6Al-4V ELI sicher für die Langzeitimplantation im menschlichen Körper?
Antwort: Ja, absolut. Bei korrekter Herstellung gilt 3D-gedrucktes Ti-6Al-4V ELI (Grad 23) als sehr sicher für die Langzeitimplantation. Hier ist der Grund:
- Material Biokompatibilität: Ti-6Al-4V ELI selbst hat aufgrund seiner hervorragenden Biokompatibilität, Korrosionsbeständigkeit und günstigen mechanischen Eigenschaften eine lange und erfolgreiche Geschichte in medizinischen Implantaten. Es entspricht strengen internationalen Normen wie ASTM F136 (“Standard Specification for Wrought Titanium-6Aluminum-4Vanadium ELI (Extra Low Interstitial) Alloy for Surgical Implant 1 Anwendungen”). Während sich diese Norm ursprünglich auf Knetwerkstoffe bezog, bilden die chemische Zusammensetzung und die Biokompatibilitätskriterien die Grundlage. Spezielle Normen für die additive Fertigung von Ti-6Al-4V (wie ASTM F3001 für PBF) gewährleisten, dass AM-Teile ähnliche Kriterien erfüllen. 1. steelplates.in steelplates.in
- Validierte Prozesse: Die Sicherheit hängt von der Verwendung hochreiner, zertifizierter Pulver in medizinischer Qualität und der Anwendung streng validierter Herstellungsverfahren ab. Dazu gehören optimierte Druckparameter, um eine nahezu vollständige Dichte zu erreichen, obligatorische Wärmebehandlungen (wie Spannungsabbau und HIP), um eine angemessene Mikrostruktur und mechanische Integrität zu gewährleisten, sowie validierte Reinigungsverfahren zur Entfernung von Restverunreinigungen.
- Regulatorische Aufsicht: Medizinprodukte, einschließlich 3D-gedruckter Implantate, unterliegen einer strengen behördlichen Aufsicht (z. B. durch die FDA in den USA oder im Rahmen der MDR in Europa). Die Hersteller müssen die Sicherheit und Wirksamkeit durch umfangreiche Tests, Prozessvalidierung und die Einhaltung von Qualitätsmanagementsystemen wie ISO 13485 nachweisen.
- Klinische Evidenz: Es gibt immer mehr klinische Belege für den erfolgreichen Einsatz von 3D-gedruckten ELI-Implantaten aus Ti-6Al-4V bei verschiedenen orthopädischen Anwendungen, einschließlich kraniofazialer Rekonstruktion und Frakturfixierung, mit hervorragenden Ergebnissen für die Patienten.
Zusammenfassend lässt sich sagen, dass das Material selbst nachweislich sicher ist, und in Kombination mit kontrollierten, validierten additiven Herstellungs- und Nachbearbeitungsabläufen erfüllen 3D-gedruckte Ti-6Al-4V ELI-Implantate die hohen Sicherheitsstandards, die für den medizinischen Einsatz erforderlich sind.
2. Wie hoch sind die Kosten für eine individuell gedruckte 3D-Knochenplatte im Vergleich zu einer Standardplatte von der Stange?
Antwort: Für einen Kostenvergleich muss man nicht nur den Preis des Implantats selbst betrachten.
- Pro-Implantat-Kosten: Eine individuelle, patientenspezifische 3D-gedruckte Knochenplatte hat im Allgemeinen eine höhere per-implantär herstellungskosten als eine in Massenproduktion hergestellte Standardplatte von der Stange. Der Grund dafür ist die Zeit, die für die Konstruktion aufgewendet werden muss, die einzigartige Maschineneinrichtung für die potenzielle Produktion aus einem Stück und die oft komplexere Geometrie, die eine sorgfältige Nachbearbeitung erfordert.
- Gesamtkosten des Verfahrens & Wert: Der Nutzen von individuell angefertigten Platten liegt oft in den potenziellen Einsparungen während der gesamten Behandlung:
- Verkürzte Zeit im Operationssaal (OR): Wenn Chirurgen Standardplatten während der Operation nicht mehr manuell biegen müssen, kann die OP-Zeit erheblich verkürzt werden. Die OP-Zeit ist extrem teuer (sie kostet viele Dollar/Euro pro Minute), so dass selbst eine Verkürzung um 20-30 Minuten zu erheblichen Kosteneinsparungen führen kann.
- Verbesserte chirurgische Ergebnisse: Die präzise anatomische Passform kann zu einer stabileren Fixierung, einer potenziell schnelleren Heilung, einem geringeren Risiko von Komplikationen (wie Malunion oder Infektion) und potenziell weniger Revisionseingriffen führen. Die Vermeidung von Komplikationen und Revisionen bedeutet langfristig erhebliche Kosteneinsparungen und eine verbesserte Lebensqualität der Patienten.
- Reduzierte Bestände: Krankenhäuser und Händler können die Kosten für die Verwaltung großer Lagerbestände von Standardplatten in zahlreichen Größen reduzieren und den Abfall durch abgelaufene oder veraltete Bestände minimieren.
- Komplexe Fälle: In hochkomplexen Fällen, in denen Standardplatten eine schlechte Lösung darstellen, können maßgefertigte Platten die einzige praktikable Option sein oder ein ansonsten schwieriges Verfahren erheblich vereinfachen und einen Wert bieten, der sich nur schwer allein anhand der Implantatkosten quantifizieren lässt.
Schlussfolgerung: Zwar sind die anfänglichen Kosten für das Implantat höher, doch der Gesamtwert, der sich aus der verkürzten OP-Zeit, den besseren Ergebnissen und der Optimierung des Lagerbestands ergibt, macht individuell gedruckte 3D-Platten oft zu einer kosteneffizienten Lösung, insbesondere bei komplexen, nicht tragenden Anwendungen.
3. Welche Informationen werden in der Regel benötigt, um eine individuelle 3D-gedruckte Knochenplatte zu bestellen?
Antwort: Die Bestellung einer patientenindividuellen Knochenplatte erfordert bestimmte Eingaben, um den Entwurfs- und Herstellungsprozess zu ermöglichen:
- Hochauflösende medizinische Bildgebungsdaten: Dies ist die Grundlage. Ein qualitativ hochwertiger CT-Scan (Computertomographie) der relevanten Anatomie des Patienten ist in der Regel erforderlich. Der Scan sollte bestimmten Protokollen in Bezug auf die Schichtdicke (in der Regel 1 mm oder weniger) und die Auflösung folgen, um eine ausreichende Detailgenauigkeit für eine genaue 3D-Modellrekonstruktion zu gewährleisten. Die Daten werden in der Regel im DICOM-Format bereitgestellt. In einigen Fällen können auch MRT-Daten verwendet werden.
- Chirurgischer Plan/Verordnung: Informationen des Chirurgen über die klinische Notwendigkeit, die Lage und Art der Fraktur oder des Defekts, die gewünschten Fixierungspunkte, kritische anatomische Strukturen, die vermieden werden sollen, und spezifische Anforderungen an die Platte (z. B. gewünschtes Profil, Bedarf an porösen Strukturen).
- Kollaborativer Designprozess: In der Regel findet ein interaktiver Prozess zwischen dem Chirurgen und dem Konstrukteur (bei dem Medizintechnikunternehmen oder dem AM-Dienstleister) statt. Dazu gehört die Überprüfung des anfänglichen anatomischen Modells, der Vorschlag eines Plattendesigns, die virtuelle Simulation der Schraubenplatzierung, die notwendigen Überarbeitungen auf der Grundlage des Feedbacks des Chirurgen und die endgültige Genehmigung des Designs durch den Chirurgen, bevor die Herstellung beginnt.
- Demografische Daten des Patienten (wenn möglich anonymisiert): Grundlegende Informationen können für die Identifizierung und Rückverfolgbarkeit durch den Produktionsprozess erforderlich sein, wobei strenge Datenschutzbestimmungen (z. B. HIPAA, GDPR) einzuhalten sind. Häufig werden eindeutige Identifikatoren verwendet.
- Bestellung / Handelsvertrag: Standard-Handelsdokumentation, in der die Bedingungen, Preise und Lieferanforderungen festgelegt sind.
Eine klare Kommunikation und die Bereitstellung vollständiger, qualitativ hochwertiger Eingabedaten sind entscheidend für die effiziente und genaue Herstellung eines individuellen Implantats.
Schlussfolgerung: Die Zukunft der orthopädischen Implantate mit Metall-3D-Druck und Met3dp
Die Integration der additiven Fertigung von Metallen in die orthopädische Chirurgie, insbesondere für nicht-lasttragende Anwendungen wie maßgeschneiderte Knochenplatten, stellt einen bedeutenden Fortschritt in der Patientenversorgung dar. Indem wir die Möglichkeiten des 3D-Drucks mit fortschrittlichen Materialien wie Ti-6Al-4V ELI nutzen, überwinden wir die Grenzen herkömmlicher, standardisierter Implantate und bewegen uns auf eine Zukunft der wirklich personalisierten Medizin zu.
Die Vorteile sind überzeugend und klar:
- Unerreichte anatomische Passform: Patientenspezifische Platten, die aus CT-Daten abgeleitet werden, bieten eine bessere Konformität, was zu erhöhter Stabilität, potenziell schnellerer Heilung und besseren funktionellen und ästhetischen Ergebnissen führt, insbesondere bei komplexen kraniofazialen und maxillofazialen Rekonstruktionen.
- Gestaltungsfreiheit für optimale Funktion: AM ermöglicht die Herstellung komplizierter Geometrien, einschließlich optimierter Formen, variabler Dicken und poröser Strukturen, die die Osseointegration fördern und zu einer besseren langfristigen biologischen Fixierung führen.
- Operative Effizienz: Vorgeformte individuelle Platten reduzieren die intraoperative Zeit, die für das Biegen von Implantaten aufgewendet werden muss, erheblich, was wertvolle OP-Zeit spart, die Anästhesiebelastung verringert und möglicherweise die Gesamtkosten des Verfahrens senkt.
- Lösungen für komplexe Fälle: Diese Technologie bietet effektive Lösungen für schwierige anatomische Variationen oder Defekte, bei denen Standardimplantate versagen.
Die Verwirklichung dieser Vorteile erfordert jedoch in jeder Phase ein Engagement für Spitzenleistungen. Dies erfordert qualitativ hochwertige Eingabedaten, ein sorgfältiges Design auf der Grundlage der DfAM-Grundsätze, die Verwendung zertifizierter medizinischer Materialien wie Ti-6Al-4V ELI und streng kontrollierte und validierte Fertigungsprozesse - vom Druck über die Wärmebehandlung, HIP, Endbearbeitung und Reinigung. Die Auswahl eines fähigen und konformen Fertigungspartners mit nachgewiesener Erfahrung in der Medizintechnik und einem robusten, nach ISO 13485 zertifizierten Qualitätssystem ist von entscheidender Bedeutung.
Die Zukunft der orthopädischen Implantate liegt zweifellos in einer stärkeren Personalisierung, die digitale Arbeitsabläufe und eine fortschrittliche Fertigung nutzt. Wir stellen uns eine Landschaft vor, in der Chirurgen routinemäßig maßgeschneiderte Implantate bestellen können, die genau auf die individuellen Bedürfnisse jedes Patienten zugeschnitten sind, was zu besser vorhersehbaren und erfolgreicheren Operationen führt. Der 3D-Metalldruck ist die zentrale Technologie, die diesen Wandel ermöglicht.
Unternehmen wie Met3dp stehen bei dieser Entwicklung an vorderster Front. Met3dp verfügt über umfassendes Know-how in der Herstellung von leistungsstarken, sphärischen Metallpulvern, die für AM optimiert sind, sowie in der Entwicklung von branchenführenden SEBM-Drucksystemen (Selective Electron Beam Melting) und bietet umfassende Lösungen für die Medizintechnikbranche. Unser Engagement für Qualität, Innovation und Prozesskontrolle stellt sicher, dass unsere Partner Zugang zu den Materialien und Technologien haben, die für die Herstellung sicherer, zuverlässiger und effektiver 3D-gedruckter medizinischer Implantate erforderlich sind. Wir arbeiten mit Medizinprodukteherstellern, Forschungseinrichtungen und Großhandelslieferanten zusammen, um die Einführung der additiven Fertigung zu beschleunigen und ihr volles Potenzial auszuschöpfen.
Wenn Sie mehr darüber erfahren möchten, wie Met3dp’s fortschrittliche Metallpulver und SEBM-Drucklösungen Ihr Unternehmen auf dem Weg in die Zukunft der personalisierten orthopädischen Implantate unterstützen können, wenden Sie sich bitte an Kontaktieren Sie uns um Ihre spezifischen Anforderungen zu besprechen. Lassen Sie uns gemeinsam die Zukunft der Medizinprodukteherstellung gestalten.
Teilen auf
MET3DP Technology Co., LTD ist ein führender Anbieter von additiven Fertigungslösungen mit Hauptsitz in Qingdao, China. Unser Unternehmen ist spezialisiert auf 3D-Druckgeräte und Hochleistungsmetallpulver für industrielle Anwendungen.
Fragen Sie an, um den besten Preis und eine maßgeschneiderte Lösung für Ihr Unternehmen zu erhalten!
Verwandte Artikel
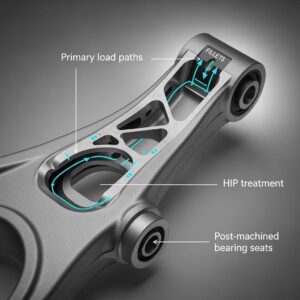
Metal 3D Printing for U.S. Automotive Lightweight Structural Brackets and Suspension Components
Mehr lesen "Über Met3DP
Aktuelles Update
Unser Produkt
KONTAKT US
Haben Sie Fragen? Senden Sie uns jetzt eine Nachricht! Wir werden Ihre Anfrage mit einem ganzen Team nach Erhalt Ihrer Nachricht bearbeiten.
Holen Sie sich Metal3DP's
Produkt-Broschüre
Erhalten Sie die neuesten Produkte und Preislisten