Patient-Specific Orthopedic Implants via Metal 3D Printing
Table of Contents
Revolutionizing Orthopedics: The Power of Metal 3D Printing for Patient-Specific Implants
The field of orthopedic surgery stands on the cusp of a profound transformation, driven by the convergence of advanced medical imaging, computational design, and groundbreaking manufacturing technologies. For decades, surgeons and patients have largely relied on standardized, off-the-shelf implants available in a limited range of sizes. While these have brought relief to millions, they represent a compromise – an approximation of fit rather than a true anatomical match. This one-size-fits-many approach can lead to challenges: longer and more complex surgeries, potential mismatches requiring intraoperative adjustments, increased bone resection, suboptimal load distribution, and, in some cases, reduced implant longevity or less-than-ideal functional outcomes. The inherent variability in human anatomy means that a standard implant, no matter how well-designed, cannot perfectly cater to every individual’s unique skeletal structure, especially in complex cases involving trauma, disease, or revision surgeries.
Enter the era of patient-specific implants (PSIs), a cornerstone of personalized medicine in orthopedics. PSIs are medical devices meticulously designed and fabricated to match an individual patient’s unique anatomy, derived typically from high-resolution imaging data like Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) scans. This shift from standardized components to bespoke solutions promises not only a superior anatomical fit but also the potential for improved surgical precision, reduced operating times, better biomechanical performance, and ultimately, enhanced patient recovery and long-term well-being.
The key enabling technology behind this revolution is metal additive manufacturing (AM), commonly known as metal 3D printing. Unlike traditional subtractive manufacturing (machining away material from a solid block) or formative manufacturing (casting or forging), additive manufacturing builds parts layer by layer directly from a digital 3D model, fusing fine metal powder particles together using a high-energy source like a laser or an electron beam. This layer-wise construction process unlocks unprecedented design freedom, making it possible to create highly complex geometries, intricate internal structures, and precisely tailored forms that are difficult, if not impossible, to achieve using conventional methods.
For orthopedic devices, metal AM offers a powerful toolkit:
- Unmatched Customization: Ability to translate patient-specific CT/MRI data into perfectly fitting implants.
- Complex Geometries: Creation of sophisticated designs, including optimized porous structures that encourage bone ingrowth (osseointegration).
- Material Efficiency: Reduced material waste compared to subtractive methods, particularly important for expensive biocompatible alloys like titanium and cobalt-chrome.
- Potential for Faster Turnaround: Streamlined digital workflow from scan to implant can potentially shorten lead times for custom devices.
- Functional Integration: Ability to incorporate features directly into the implant design, potentially consolidating multiple components into a single, stronger part.
This medical 3D printing introduction merely scratches the surface of AM’s potential in orthopedics. As the technology matures and adoption grows, we are witnessing a fundamental change in how orthopedic implants are conceived, designed, manufactured, and utilized. It represents a move towards truly personalized surgical solutions, where the implant is no longer a limiting factor but an enabler of better patient care. Companies specializing in metal AM, equipped with advanced printing technologies and expertise in processing medical-grade materials, are pivotal in translating this potential into clinical reality, providing surgeons and medical device manufacturers with the tools needed to deliver the next generation of orthopedic care. The journey involves close collaboration between surgeons, engineers, and metal additive manufacturing suppliers to ensure that these advanced patient-specific implants meet the rigorous demands of clinical application.
Applications of Custom Metal 3D Printed Orthopedic Implants
The versatility and precision of metal additive manufacturing have unlocked a wide array of applications within orthopedics, moving far beyond niche uses into mainstream surgical practice for complex cases and increasingly for primary procedures. The ability to create custom orthopedic implants tailored to individual anatomy and surgical needs is proving invaluable across various specialties. Here are some key areas where metal 3D printing is making a significant impact:
1. Joint Replacement (Arthroplasty) – Hip, Knee, Shoulder:
- Acetabular Cups (Hip): Metal AM excels in creating hip sockets with integrated porous structures (trabecular or lattice designs) on the outer surface. These biomimetic structures mimic cancellous bone, promoting superior biological fixation through bone ingrowth, potentially leading to longer implant survival compared to traditional press-fit or cemented cups with sintered bead or plasma spray coatings. Custom cups can also address significant bone defects or unusual pelvic anatomy, particularly in revision hip surgery.
- Femoral Components (Hip & Knee): While less common for standard primary components due to established traditional manufacturing efficiencies, AM is used for custom femoral stems or knee components in cases of significant deformity, bone loss, or unusual patient anatomy where off-the-shelf options would be inadequate. Patient-specific femoral stems can optimize load transfer and potentially reduce stress shielding.
- Tibial Trays & Augments (Knee): Similar to acetabular cups, AM allows for integrated porous structures for enhanced fixation. Custom tibial baseplates and augments are particularly useful in revision knee arthroplasty to reconstruct bone defects and restore joint line height accurately.
- Glenoid Components (Shoulder): Reverse shoulder arthroplasty often involves glenoid bone loss. Custom 3D printed glenoid baseplates, sometimes with integrated augments, can provide a stable foundation matched precisely to the patient’s remaining bone stock, which is challenging with standard components.
- Keywords Focus: 3D printed joint replacements, custom hip implants AM, patient-specific knee components, orthopedic implant use cases, revision arthroplasty solutions.
2. Spinal Surgery:
- Interbody Fusion Cages: This is one of the most rapidly growing applications. AM enables the creation of spinal cages (used in procedures like TLIF, PLIF, ALIF) with precisely controlled porosity (both surface and internal architecture) and optimized shapes that conform perfectly to the patient’s vertebral endplates. The porous structure enhances osseointegration for a solid fusion, while the customized shape maximizes contact area and stability. Materials like Ti-6Al-4V ELI are preferred for their biocompatibility and imaging characteristics. Topology optimization can also be used to create lightweight yet strong cage designs.
- Vertebral Body Replacements (VBRs): In cases of tumor resection, trauma, or severe deformity requiring removal of one or more vertebral bodies, custom 3D printed VBRs can be designed to perfectly span the defect and restore spinal column stability and alignment. AM allows for complex shapes and integrated fixation features.
- Patient-Specific Pedicle Screw Guides: While not implants themselves, 3D printed surgical guides, often made from polymers but sometimes metal for specific applications, assist surgeons in accurately placing pedicle screws, especially in deformed spines.
- Keywords Focus: Spinal fusion cages AM, 3D printed spine implants, custom interbody devices, vertebral body replacement AM, orthopedic implant use cases.
3. Cranio-Maxillofacial (CMF) Reconstruction:
- Patient-Specific Cranial Plates: Following trauma, tumor resection, or decompressive craniectomy, large or complex skull defects can be precisely reconstructed using custom-designed CMF plates, typically made from Ti-6Al-4V ELI. These plates provide protection and restore cranial contour with excellent cosmetic results, far superior to hand-bent mesh or pre-formed plates.
- Mandibular/Maxillary Reconstruction Plates: AM allows for the creation of intricate plates that perfectly match the contours of the jawbone, essential for restoring function (chewing, speaking) and aesthetics after significant trauma or cancer surgery involving bone removal. Designs can incorporate features to guide bone grafts or integrate with dental implants.
- Orbital Floor/Wall Implants: Custom implants can reconstruct the delicate structures of the eye socket after fractures, ensuring correct eye position and volume.
- Keywords Focus: CMF implants manufacturing, patient-specific cranial plates, custom jaw reconstruction, 3D printed facial implants, maxillofacial surgery AM.
4. Trauma Fixation:
- Complex Fracture Plates: For highly comminuted (fragmented) or periarticular fractures (near a joint) with unusual patterns, standard plates may not provide adequate fixation or fit well. Custom 3D printed plates, designed based on the patient’s specific fracture pattern derived from CT scans, can offer superior stability and anatomical reduction.
- Intramedullary Nails: While less common, custom nails might be considered for long bones with extreme bowing or very narrow/wide canals.
- Pelvic Fracture Fixation: Complex pelvic ring injuries often require intricate fixation. Custom plates designed using AM can potentially simplify these challenging surgeries and provide better stability.
- Keywords Focus: Trauma fixation devices, custom fracture plates, 3D printed orthopedic implants, complex trauma solutions AM.
5. Limb Salvage and Oncology:
- Tumor Resection Implants: When bone tumors necessitate large resections, custom 3D printed implants can replace the removed bone segment, preserving the limb. These often feature complex geometries and porous sections for integration with remaining bone or soft tissues.
- Osteotomy Guides and Implants: For corrective osteotomies (cutting and realigning bone), custom implants and associated surgical guides designed via AM ensure high precision.
The breadth of these applications underscores the adaptability of metal additive manufacturing. It empowers surgeons and medical device companies to move beyond the limitations of standard inventory and provide tailored solutions, particularly for complex orthopedic challenges where anatomical fit and biological integration are paramount.

Why Metal Additive Manufacturing is Ideal for Orthopedic Implant Production
The rapid adoption of metal additive manufacturing in orthopedics isn’t just driven by novelty; it’s fueled by tangible advantages over traditional manufacturing methods like machining, casting, and forging, particularly when producing complex or custom orthopedic implants. Understanding these benefits is crucial for engineers and procurement managers evaluating manufacturing options for medical devices.
Here’s a breakdown of why AM stands out:
1. Unparalleled Design Freedom & Complexity:
- AM: Builds parts layer by layer, allowing for highly intricate internal and external geometries, undercuts, internal channels, and complex curves that are impossible or prohibitively expensive to create with traditional methods. This enables:
- Biomimetic Porous Structures: Creation of lattice or trabecular structures mimicking natural bone (e.g., cancellous bone architecture) to encourage osseointegration. Porosity, pore size, and interconnectivity can be precisely controlled.
- Topology Optimization: Algorithmic design processes can remove material from low-stress areas, resulting in implants that are significantly lighter yet retain necessary strength, improving biomechanical compatibility (e.g., reducing stress shielding).
- Anatomical Matching: Direct translation of patient CT/MRI data into perfectly contoured implant shapes for a precise fit.
- Traditional: Limited by tool access (machining) or mold/die constraints (casting/forging). Complex internal features are often impossible. Creating porous surfaces typically requires secondary coating processes (e.g., plasma spray, sintered beads), which offer less control over structure compared to AM.
2. Enhanced Osseointegration Potential:
- AM: Enables the integration of porous structures directly into the solid implant body as a single piece. This eliminates potential interface failures associated with coatings and allows for complex, optimized pore architectures designed to maximize bone ingrowth and long-term biological fixation.
- Traditional: Relies on surface treatments or coatings applied after the main implant body is formed. While effective, these coatings have limitations in terms of structural complexity, adhesion strength, and potential for delamination or particle shedding over time.
3. Suitability for Patient-Specific Implants (PSIs):
- AM: Ideal for low-volume, high-complexity parts. The digital workflow (scan-to-design-to-print) is highly adaptable for creating unique, custom implant advantages. Tooling is minimal (digital file), making one-off production economically viable compared to traditional methods requiring custom molds or complex machining setups.
- Traditional: Primarily geared towards mass production of standardized parts. Creating custom implants requires significant setup time, specialized tooling (molds, jigs, fixtures), and extensive programming, making it costly and time-consuming for single or small batches.
4. Material Efficiency:
- AM (Powder Bed Fusion): Uses only the material needed for the part and necessary support structures. Unfused powder can often be recycled and reused (with proper quality control), significantly reducing waste. This is a major cost benefit when working with expensive medical-grade alloys like Ti-6Al-4V ELI or CoCrMo.
- Traditional (Machining): Subtractive nature means significant amounts of material are cut away and become scrap (chips), often representing >50-80% of the initial raw material block for complex parts. While chips can be recycled, the initial material cost and processing time are higher. Casting can be more efficient but often requires significant machining afterwards.
5. Part Consolidation:
- AM: Complex assemblies that traditionally consist of multiple components requiring joining (welding, brazing, fasteners) can often be redesigned and printed as a single, monolithic part. This reduces assembly time, potential points of failure, and overall weight.
- Traditional: Limited ability to consolidate complex parts; assemblies are often necessary.
6. Potential for Lead Time Reduction (for Custom Parts):
- AM: Once the design is finalized, printing can often commence relatively quickly without the need for creating physical tooling. While printing and post-processing take time, the overall lead time for a custom implant can potentially be shorter than sourcing custom tooling and machining/casting, especially for urgent cases. This aids in supply chain optimization medical.
- Traditional: Tooling creation for custom parts can take weeks or months, significantly extending lead times.
Comparison Summary: AM vs. Traditional Manufacturing for Orthopedic Implants
| Feature | Metal Additive Manufacturing (AM) | Traditional Manufacturing (Machining, Casting) |
|---|---|---|
| Design Complexity | Very High (complex geometries, internal features, lattices) | Limited by tool access or mold constraints |
| Customization (PSI) | Highly suitable, economically viable for single parts | Costly and time-consuming for single parts due to tooling |
| Porous Structures | Integrated, highly controllable (biomimetic) | Typically surface coatings (less control, potential interface issues) |
| Osseointegration | Enhanced potential via engineered porosity | Reliant on surface treatments/coatings or press-fit stability |
| Material Waste | Low (powder recycling possible) | High (machining) or Moderate (casting + finishing) |
| Part Consolidation | High potential | Low potential |
| Lead Time (Custom) | Potentially faster (no hard tooling) | Longer (tooling development required) |
| Ideal Volume | Low-to-Medium Volume, High Complexity/Customization | High Volume, Standardized Parts |
Export to Sheets
While traditional manufacturing remains highly efficient for mass-producing standard implants, metal additive manufacturing offers compelling, often essential, advantages for the growing demand for patient-specific implants, complex reconstructions, and devices featuring advanced designs for improved biological fixation. The benefits of 3D printing implants align perfectly with the goals of personalized medicine and advanced orthopedic care.
Biocompatible Metal Powders for 3D Printed Orthopedic Implants: Ti-6Al-4V ELI & CoCrMo
The success of any 3D printed orthopedic implant hinges critically on the material used. Implants reside within the human body for potentially decades, interacting constantly with biological tissues and fluids under significant mechanical loads. Therefore, the materials must meet stringent requirements for biocompatibility, mechanical strength, corrosion resistance, and long-term stability. For load-bearing orthopedic applications produced via metal AM, two families of alloys dominate: Titanium alloys (specifically Ti-6Al-4V ELI) and Cobalt-Chromium-Molybdenum (CoCrMo) alloys.
Selecting the appropriate biocompatible metal powders and ensuring their quality is paramount. This is where partnering with an experienced material and AM provider like Met3dp becomes crucial. Met3dp utilizes advanced powder production techniques, including industry-leading gas atomization and Plasma Rotating Electrode Process (PREP) technologies, to manufacture high-quality metal powders with characteristics optimized for additive manufacturing processes like Selective Electron Beam Melting (SEBM) and laser powder bed fusion (L-PBF/DMLS/SLM).
1. Titanium Alloy: Ti-6Al-4V ELI (Grade 23)
- Composition: Primarily Titanium (Ti), with ~6% Aluminum (Al) and ~4% Vanadium (V). The “ELI” designation stands for Extra Low Interstitials, meaning it has strictly controlled lower limits for elements like oxygen, nitrogen, carbon, and iron compared to standard Grade 5 Ti-6Al-4V.
- Key Properties:
- Excellent Biocompatibility: Forms a stable, passive oxide layer (TiO2) upon exposure to air or bodily fluids, minimizing ion release and adverse tissue reactions. Widely accepted as safe for long-term implantation.
- High Strength-to-Weight Ratio: Offers strength comparable to many steels but at nearly half the density. This reduces implant weight and can be advantageous biomechanically.
- Superior Corrosion Resistance: Highly resistant to corrosion in the aggressive chemical environment of the human body.
- Good Fatigue Strength: ELI grade offers improved ductility and fracture toughness compared to standard Grade 5, critical for implants under cyclic loading.
- Lower Modulus of Elasticity: Its stiffness (modulus) is closer to that of natural bone (~110 GPa for Ti-6Al-4V vs. ~10-30 GPa for bone) compared to CoCrMo or stainless steel. This can reduce “stress shielding,” where the stiff implant carries too much load, leading to bone density loss around it.
- MRI Compatibility: Generally considered safe for MRI scans, although some imaging artifacts may occur.
- Relevant Standard: ASTM F136 – “Standard Specification for Wrought Titanium-6Aluminum-4Vanadium ELI (Extra Low Interstitial) Alloy for Surgical Implant 1 Applications.” While originally for wrought material, its chemical composition and mechanical property requirements are the benchmark for AM Ti-6Al-4V ELI implants. AM-specific standards (e.g., ASTM F3001 for Ti-6Al-4V ELI via L-PBF) also apply. 1. steelplates.in steelplates.in
- Common AM Applications: Hip stems, acetabular cups, knee components, spinal fusion cages, CMF plates, trauma plates. The combination of biocompatibility, mechanical properties, and suitability for creating porous structures makes it the workhorse material for many orthopedic grade titanium AM implants.
2. Cobalt-Chromium-Molybdenum Alloy (CoCrMo)
- Composition: Primarily Cobalt (Co), with significant amounts of Chromium (Cr) (~26-30%) and Molybdenum (Mo) (~5-7%), plus minor alloying elements.
- Key Properties:
- Excellent Wear Resistance: Historically valued for its high hardness and ability to resist wear, making it a traditional choice for the bearing surfaces (metal-on-polyethylene or metal-on-metal) in hip and knee replacements.
- High Strength & Hardness: Possesses very high ultimate tensile strength and yield strength, suitable for demanding load-bearing applications.
- Very Good Corrosion Resistance: The high chromium content forms a robust passive oxide layer, providing excellent protection against corrosion in vivo.
- Established Biocompatibility: Long history of use in orthopedic implants. However, concerns exist regarding the potential release of Cobalt and Chromium ions, particularly from wear debris in metal-on-metal bearings, which has led to decreased use in those specific applications but it remains relevant for other implant types.
- Relevant Standard: ASTM F1537 – “Standard Specification for Wrought Cobalt-28Chromium-6Molybdenum Alloy for Surgical Implants (UNS R31537, R31538, and R31539).” Similar to Ti-6Al-4V ELI, this standard for wrought material often serves as a reference for AM CoCrMo implants, alongside AM-specific standards like ASTM F3426.
- Common AM Applications: While less common now for bearing surfaces due to ion concerns, AM CoCrMo is still used for femoral components in knee replacements (due to wear against polyethylene tibial inserts), some spinal applications, CMF implants, and dental frameworks/implants where high strength and wear resistance are critical.
The Critical Role of Powder Quality:
Regardless of the chosen alloy, the quality of the metal powder feedstock is non-negotiable for producing reliable, high-performance medical implants. Key powder characteristics influencing the final printed part include:
- Particle Size Distribution (PSD): Affects powder bed density, flowability, and the resolution of fine features. Must be tightly controlled and suited to the specific AM process (e.g., SEBM often uses coarser powder than L-PBF).
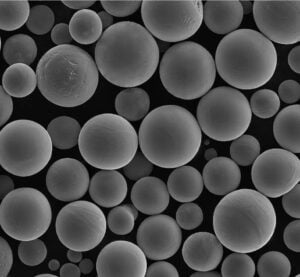
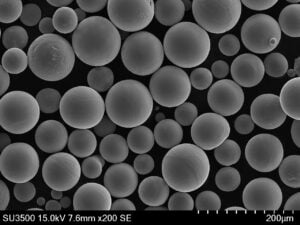
- Sphericity & Morphology: Highly spherical particles generally lead to better powder flow and denser packing in the powder bed, contributing to consistent melting and reduced porosity in the final part. Met3dp’s gas atomization process is designed to produce highly spherical powders.
- Flowability: Essential for uniformly spreading thin layers of powder during the printing process. Poor flowability can lead to defects.
- Chemical Purity: Must meet the stringent requirements of medical standards (e.g., ASTM F136, F1537), with minimal impurities and controlled levels of interstitial elements like oxygen and nitrogen, which can significantly impact mechanical properties and biocompatibility.
- Absence of Satellites: Small particles attached to larger ones (“satellites”) can hinder flowability and packing density. Advanced processes like PREP, employed by Met3dp, can produce highly spherical powders with fewer satellites.
- Batch-to-Batch Consistency: Reliable Ti-6Al-4V ELI powder suppliers and CoCrMo implant material providers must ensure consistent properties across different powder batches for predictable manufacturing outcomes.
Material Properties Summary:
| Property | Ti-6Al-4V ELI (Grade 23) | CoCrMo Alloy (ASTM F1537) | Notes |
|---|---|---|---|
| Primary Composition | Ti (~89%), Al (~6%), V (~4%) | Co (Balance), Cr (~28%), Mo (~6%) | Minor elements controlled per standards. |
| Density | ~4.43 g/cm³ | ~8.3 g/cm³ | Ti-6Al-4V ELI is significantly lighter. |
| Biocompatibility | Excellent | Good (ion release concerns in wear) | Both widely used, Ti often preferred where ion release is a concern. |
| Corrosion Resistance | Excellent | Excellent | Both form stable passive layers. |
| Elastic Modulus | ~110-115 GPa | ~210-230 GPa | Ti-6Al-4V ELI closer to bone (~10-30 GPa), reducing stress shielding. |
| Yield Strength (Typical) | >760-830 MPa (Annealed/HIPed) | >500-650 MPa (Annealed/HIPed) | Properties depend heavily on post-processing (heat treat, HIP). |
| Ultimate Tensile Strength | >860-900 MPa (Annealed/HIPed) | >800-1000 MPa (Annealed/HIPed) | CoCrMo can achieve higher ultimate strength. |
| Wear Resistance | Moderate | Excellent | CoCrMo superior for bearing surfaces (if used). |
| Governing Standard (Ref) | ASTM F136 | ASTM F1537 | AM-specific standards also apply (F3001, F3426, etc.). |
Export to Sheets
Met3dp’s commitment to producing high-quality metal powders using advanced techniques like gas atomization and PREP, combined with rigorous quality control, ensures that manufacturers of orthopedic implants have access to reliable, high-performance materials tailored for the demanding requirements of additive manufacturing and clinical application. Partnering with a knowledgeable material supplier is the first step toward successful metal AM implant production. Explore Met3dp’s range of high-quality metal powders to learn more.

Design for Additive Manufacturing (DfAM) Principles for Optimal Orthopedic Implants
Transitioning from traditional manufacturing paradigms to metal additive manufacturing requires more than just converting a standard CAD file into a printable format. To truly harness the power of AM and produce high-quality, functional, and cost-effective orthopedic implants, engineers and designers must embrace Design for Additive Manufacturing (DfAM) principles. DfAM is not merely a suggestion; it’s a fundamental necessity for success in designing for metal 3D printing. It involves rethinking part design from the ground up to leverage AM’s unique capabilities while mitigating its inherent constraints. Applying DfAM specifically for medical devices like orthopedic implants ensures optimal structural integrity, biocompatibility, manufacturability, and clinical performance.
Key DfAM principles for metal AM orthopedic implants include:
1. Strategic Part Orientation:
- Impact: The orientation of the implant on the build plate significantly influences several critical factors:
- Support Structures: Affects the amount and location of required supports. Orienting to minimize overhangs reduces support needs.
- Surface Finish: Surfaces facing upwards or downwards during the build often have different roughness characteristics. Critical surfaces may dictate orientation choices.
- Mechanical Properties: Layer-by-layer building can sometimes lead to slight variations in material properties depending on the build direction (anisotropy), although post-processing like HIP significantly minimizes this.
- Build Time & Cost: Taller parts generally take longer to print. Packing multiple parts efficiently on a build plate also depends on individual part orientation.
- Consideration: Analyze the implant geometry to find an orientation that balances minimal support needs, optimal surface finish on critical areas, potentially favorable mechanical loading direction, and efficient build time.
2. Support Structure Strategy:
- Purpose: Support structures are crucial in powder bed fusion processes (like SEBM and L-PBF) for several reasons:
- Supporting Overhanging Features: Metal AM typically requires supports for features angled below a certain threshold (often ~45 degrees) from the horizontal plane to prevent collapse or deformation.
- Anchoring the Part: Secures the part to the build plate, preventing warping due to thermal stresses.
- Heat Dissipation: Conducts heat away from the melt pool, especially for fine features or overhangs, ensuring proper solidification.
- DfAM Approach:
- Minimize Supports: Design with self-supporting angles (greater than ~45 degrees) wherever possible. Use fillets instead of sharp horizontal overhangs.
- Optimize Support Design: Utilize software tools to generate efficient support structures (e.g., lattice, block, or tree supports) that use minimal material and are easier to remove. Consider perforated or thin-contact-point supports.
- Design for Removal: This is critical and often overlooked. Ensure adequate access for tools to remove supports post-printing without damaging the implant surface. Avoid placing supports on functionally critical or complex surfaces if possible. Design breakaway points or specific features to facilitate easier removal. The support structure strategy AM must consider the entire workflow, including post-processing labor.
3. Topology Optimization:
- Concept: A powerful computational design technique where software algorithms iteratively remove material from a design space based on defined load cases, constraints, and performance targets.
- Benefit for Implants: Creates highly efficient, often organic-looking structures that are optimized for strength-to-weight ratio. This is ideal for orthopedic implants where reducing weight can minimize stress shielding (the implant carrying too much load, causing adjacent bone to weaken) while maintaining the required structural integrity. Topology optimization orthopedics leads to implants that are biomechanically more compatible.
- AM Advantage: The complex, sometimes non-intuitive geometries resulting from topology optimization are often only manufacturable using AM.
4. Lattice and Porous Structure Design:
- Purpose: One of AM’s most significant advantages for orthopedics is the ability to create integrated porous structures that mimic the trabecular architecture of natural bone, promoting osseointegration for long-term biological fixation.
- DfAM Considerations:
- Unit Cell Selection: Choose appropriate lattice unit cells (e.g., cubic, star, diamond, gyroid, stochastic) based on desired mechanical properties (stiffness, strength) and biological response.
- Porosity Control: Define the overall percentage of void space, pore size, and strut thickness. These parameters influence bone ingrowth potential and mechanical properties. Target pore sizes are often in the range of 300-800 microns for optimal bone cell infiltration.
- Interconnectivity: Ensure pores are interconnected to allow cell migration and vascularization throughout the structure.
- Cleanability: Design lattices that can be effectively cleaned post-manufacturing to remove residual powder particles, which is critical for biocompatibility. Avoid closed cells or overly complex internal pathways where powder might become trapped.
- Structural Integrity: Ensure the lattice provides sufficient mechanical support and seamlessly transitions into the solid portions of the implant.
- Keywords Focus: lattice structure design implants, porous implant structures, osseointegration enhancement.
5. Feature Resolution and Minimum Dimensions:
- Constraint: Every AM process has limitations on the smallest features it can accurately produce. This includes minimum wall thickness, smallest hole or channel diameter, minimum pin size, and sharpest edge radius.
- DfAM Approach: Consult the specifications of the chosen AM system (e.g., SEBM, L-PBF) and material. Design features comfortably above these minimum limits to ensure manufacturability and robustness. Avoid overly thin walls or delicate features prone to damage during printing or post-processing unless absolutely necessary and validated.
6. Residual Stress Management:
- Challenge: The rapid heating and cooling cycles inherent in metal AM can build up internal stresses, potentially leading to part distortion (warping) or even cracking.
- DfAM Mitigation:
- Avoid large, flat surfaces parallel to the build plate.
- Use generous radii and fillets instead of sharp corners.
- Orient the part to minimize thermal gradients.
- Incorporate stress-relief features if necessary (though often managed by supports and post-print heat treatment).
7. Designing for Post-Processing:
- Reality: Metal AM implants almost always require post-processing. DfAM must account for these subsequent steps.
- Considerations:
- Machining Allowance: Add extra material (machining stock) to surfaces requiring high precision or specific finishes (e.g., taper junctions, bearing surfaces, screw interfaces) that will be achieved via CNC machining.
- Support Removal Access: As mentioned, ensure supports can be physically reached and removed.
- Handling Features: Consider adding temporary features (lugs, tabs) to aid in handling the part during post-processing, which can be removed later.
- Inspection Access: Ensure critical features are accessible for measurement and inspection tools (e.g., CMM probes, visual inspection).
Implementing these DfAM for medical devices principles requires a collaborative approach between design engineers, AM process specialists, and manufacturing partners. It transforms the design process from simply creating a shape to engineering a comprehensive solution optimized for additive manufacturing and end-use performance.
Achieving Precision: Tolerance, Surface Finish, and Dimensional Accuracy in 3D Printed Implants
Precision is paramount in orthopedic implants. Proper fit, function, and integration with the patient’s anatomy and surgical instrumentation depend on achieving specific dimensional tolerances and surface characteristics. While metal additive manufacturing offers incredible geometric freedom, understanding its inherent precision capabilities and limitations is crucial for setting realistic expectations and planning necessary post-processing steps. The achievable metal 3D printing tolerances medical, surface finish, and overall dimensional accuracy AM parts depend on the specific AM technology used (e.g., SEBM vs. L-PBF), machine calibration, material properties, part geometry, and process parameters.
1. Dimensional Accuracy and Tolerances:
- As-Built Accuracy: Metal AM processes typically produce parts with good, but not necessarily machining-level, accuracy in their ‘as-built’ state (after printing but before major post-processing).
- Selective Electron Beam Melting (SEBM): Often operates at higher temperatures in a vacuum, which helps reduce internal stresses. Typical as-built tolerances for SEBM might be in the range of +/- 0.2 mm to +/- 0.4 mm, or a percentage of the dimension (e.g., +/- 0.5%). Met3dp utilizes industry-leading SEBM printers known for their reliability and accuracy in producing complex titanium parts. You can learn more about different printing methods like SEBM on our website.
- Laser Powder Bed Fusion (L-PBF / DMLS / SLM): Can sometimes achieve slightly tighter as-built tolerances, potentially in the range of +/- 0.1 mm to +/- 0.2 mm, depending on the machine, material, and parameters. However, L-PBF processes generally induce higher residual stresses, which can affect accuracy, especially on larger parts.
- Factors Influencing Accuracy: Thermal expansion/contraction during the build, beam spot size or laser focus, powder layer thickness, calibration accuracy, part size and geometry (larger parts or complex shapes may see more deviation), and support structure effectiveness all play a role.
- Achieving Tight Tolerances: For critical features requiring tolerances tighter than achievable ‘as-built’ (e.g., modular taper junctions, screw holes, bearing surfaces), post-process CNC machining is essential. DfAM principles dictate adding machining stock in these areas.
2. Surface Finish (Roughness):
- As-Built Surface: The layer-by-layer nature of AM and the partially melted powder particles adhering to the surface result in a characteristic roughness.
- SEBM: Typically produces rougher surfaces compared to L-PBF due to generally larger powder particle sizes and higher beam energy causing more particle sintering. As-built Ra (average roughness) values for SEBM Ti-6Al-4V ELI are often in the range of 20-35 µm.
- L-PBF: Can achieve smoother as-built surfaces, often with Ra values ranging from 5-15 µm, depending heavily on parameters, material, and surface orientation (upward-facing, downward-facing, and vertical walls will have different roughness).
- Impact of Orientation: Surfaces built parallel to the layers (top/bottom) tend to be smoother than vertical walls, which exhibit the characteristic layer stepping effect. Downward-facing surfaces relying on supports are often the roughest due to support contact points.
- Post-Processing for Smoothness: As-built surfaces are generally too rough for bearing applications or interfaces requiring smooth contact. Post-processing steps like grinding, polishing, bead blasting, or electropolishing are necessary to achieve the required surface finish orthopedic implants demand, often targeting Ra values below 1 µm or even lower for highly polished bearing surfaces. Porous surfaces intended for bone ingrowth are typically left in their rougher, as-built or lightly treated state to maximize surface area and encourage biological fixation.
3. Feature Detail and Resolution:
- Minimum Feature Size: AM processes have limits on the smallest positive features (e.g., pins, thin struts in lattices) and negative features (e.g., holes, channels) they can reliably produce. This depends on beam/laser spot size, powder particle size, and layer thickness. Minimum producible wall thicknesses might range from 0.3 mm to 0.8 mm, varying by process and machine.
- Edge Sharpness: Achieving perfectly sharp edges is difficult; edges will typically have a slight radius due to the melt pool dynamics.
4. Repeatability and Consistency:
- Importance: Ensuring that every implant produced meets the same specifications is critical for medical devices. This requires robust process control and quality management systems.
- Factors: Machine calibration, consistent powder quality (feedstock management is vital), tightly controlled process parameters (beam power, scan speed, layer thickness, temperature), and a stable operating environment contribute to repeatability. Implant quality control relies on validating the entire process chain.
Precision Comparison: SEBM vs. L-PBF (Typical Ranges for Ti-6Al-4V ELI)
| Feature | SEBM (Electron Beam) | L-PBF (Laser Beam) | Notes |
|---|---|---|---|
| As-Built Tolerance | +/- 0.2 to 0.4 mm (Typical) | +/- 0.1 to 0.2 mm (Typical) | Can vary significantly with geometry, size, parameters. |
| As-Built Ra (Avg) | 20 – 35 µm | 5 – 15 µm | Highly dependent on surface orientation and parameters. |
| Min. Wall Thickness | ~0.5 – 0.8 mm | ~0.3 – 0.5 mm | Process and machine specific. |
| Residual Stress | Lower (due to high build temp/vacuum) | Higher (requires more stress management) | Affects dimensional stability, especially pre-heat treatment. |
| Build Speed | Generally Faster (multiple beam/scan) | Generally Slower (single or few lasers) | Machine specific; SEBM often favored for larger Ti parts. |
| Powder Handling | Requires vacuum, pre-sintered cake | Inert gas atmosphere, loose powder handling | Different safety and handling protocols. |
Export to Sheets
Understanding these precision aspects allows engineers to design implants appropriately, specifying post-processing requirements accurately and selecting the most suitable EBM vs DMLS implants manufacturing technology (SEBM vs. L-PBF) based on the specific needs of the orthopedic device. Reputable service providers like Met3dp provide guidance on achievable precision with their specific equipment and processes, ensuring parts meet critical dimensional accuracy AM parts requirements.

Essential Post-Processing Steps for Metal 3D Printed Orthopedic Implants
A common misconception about metal additive manufacturing is that parts come out of the printer ready for use. For demanding applications like orthopedic implants, the printing process is just one step in a comprehensive manufacturing workflow. Post-processing 3D printed implants involves a series of crucial steps to transform the raw, as-built part into a safe, functional, and biocompatible medical device meeting stringent quality and regulatory standards. Neglecting or improperly performing these steps can compromise the implant’s mechanical integrity, surface properties, and ultimately, patient safety.
The typical post-processing workflow for metal AM orthopedic implants includes:
- Thermal Stress Relief:
- Purpose: To alleviate the internal stresses built up during the rapid heating and cooling cycles of the AM process. These stresses can cause distortion, warping, or even cracking if not addressed.
- Process: Typically performed while the part is still attached to the build plate, often inside the AM machine itself or in a separate vacuum/inert atmosphere furnace. Involves heating the part to a specific temperature below the material’s transformation point and holding it for a set duration, followed by controlled cooling. Heat treatment medical AM protocols are specific to the alloy (e.g., Ti-6Al-4V ELI, CoCrMo) and AM process used.
- Part Removal from Build Plate:
- Purpose: To separate the printed implant(s) from the metal base plate they were built upon.
- Methods: Commonly done using wire Electrical Discharge Machining (EDM) or a band saw. Care must be taken not to damage the parts during removal.
- Support Structure Removal:
- Purpose: To remove the temporary support structures required during the build process.
- Methods: This can be one of outcrops. The most labor-intensive steps, especially for complex geometries or internal supports. Methods include:
- Manual breaking/clipping (for easily accessible, light supports).
- CNC machining.
- Grinding or manual tooling.
- Sometimes electrochemical machining or etching for specific materials/geometries.
- Challenges: Incomplete removal can leave stress concentrations or undesirable surface artifacts. Aggressive removal can damage the implant surface. DfAM plays a critical role in designing supports for easier support removal metal AM.
- Hot Isostatic Pressing (HIP):
- Purpose: To eliminate internal microporosity (small voids within the material) that can remain after the AM process. HIP significantly improves density (often achieving >99.9% theoretical density), ductility, fatigue strength, and overall mechanical reliability of the implant.
- Process: Parts are placed in a specialized HIP vessel and subjected to high temperatures (below melting point) and high isostatic pressure (typically 100-200 MPa) using an inert gas like Argon. The combination of heat and pressure causes internal voids to collapse and diffusion bond, effectively eliminating them.
- Requirement: HIP for orthopedic implants, especially load-bearing ones made from Ti-6Al-4V ELI, is often considered mandatory by regulatory bodies and leading medical device manufacturers to ensure optimal fatigue life and structural integrity.
- Machining (CNC):
- Purpose: To achieve tight dimensional tolerances, specific surface finishes, and critical features that cannot be produced with sufficient accuracy by the AM process alone.
- Applications: Creating precise modular taper junctions (e.g., hip stem necks), drilling and tapping bone screw holes, finishing bearing surfaces, creating mating surfaces for assemblies, or adding identification markings. CNC machining implants is essential for functional interfaces.
- Surface Finishing:
- Purpose: To achieve the desired surface texture and roughness on different areas of the implant.
- Methods:
- Grinding/Polishing: For creating very smooth surfaces (e.g., Ra < 0.1 µm) on bearing components or areas requiring low friction.
- Abrasive Blasting (Bead/Grit Blasting): Creates a uniform matte finish, often used on non-articulating surfaces. Can also help remove loosely adhered powder particles. Choice of media is critical to avoid contamination.
- Tumbling/Vibratory Finishing: Can smooth surfaces and deburr edges, particularly for batches of smaller parts.
- Electropolishing: An electrochemical process that can smooth surfaces and improve corrosion resistance, particularly for CoCrMo or stainless steels.
- Cleaning and Passivation:
- Purpose: To remove all contaminants from the manufacturing process (residual powder, machining fluids, blasting media, handling residues) and to ensure the implant surface is biocompatible. Passivation specifically enhances the protective oxide layer on titanium and cobalt-chrome alloys, improving corrosion resistance.
- Process: Typically involves multi-stage ultrasonic cleaning baths with validated detergents and rinsing steps (e.g., using deionized water). Passivation often involves treatment with specific acids (like nitric acid for Ti alloys) under controlled conditions. Thorough validation of cleaning processes is critical to prevent toxic residues.
- Inspection and Quality Control:
- Purpose: To verify that the finished implant meets all design specifications and quality standards.
- Methods: Dimensional inspection (Coordinate Measuring Machines – CMM, 3D scanning), surface roughness measurement, visual inspection, Non-Destructive Testing (NDT) such as X-ray or CT scanning to confirm internal integrity (absence of large voids or inclusions), material certification checks, verification of cleaning and passivation effectiveness.
Each of these post-processing 3D printed implants steps requires careful planning, execution, and validation, adding significantly to the overall cost and lead time but ensuring the final product is safe and effective for clinical use.
Overcoming Challenges in Metal Additive Manufacturing for Orthopedics
While metal additive manufacturing offers transformative potential for orthopedic implants, it’s not without its challenges. Successfully implementing AM for medical device production requires a deep understanding of these hurdles and robust strategies to mitigate them. Engineers, manufacturers, and procurement managers must be aware of these potential issues when considering or specifying AM processes. Partnering with an experienced service provider like Met3dp, which possesses expertise in materials science, process optimization, and quality control, is often key to navigating these complexities.
Common metal AM challenges medical device manufacturers face include:
1. Residual Stress, Distortion, and Warping:
- Issue: The intense, localized heating and rapid cooling inherent in powder bed fusion create significant thermal gradients, leading to internal stresses within the printed part. These stresses can cause parts to distort, warp (especially large, flat sections), or even detach from the build plate or crack, particularly in materials like warping 3D printing titanium (Ti-6Al-4V ELI).
- Mitigation:
- DfAM: Designing parts to minimize stress concentrations (e.g., avoiding sharp corners, large flat areas).
- Build Strategy: Optimizing part orientation and using robust support structures to anchor the part and manage heat flow.
- Process Parameters: Fine-tuning scan strategies and energy input.
- Thermal Management: Utilizing build plate heating (common in L-PBF) or the high-temperature vacuum environment of SEBM (which inherently reduces stresses).
- Post-Processing: Performing immediate thermal stress relief cycles after the build is crucial.
2. Porosity Control and Density:
- Issue: Achieving full theoretical density (>99.9%) and eliminating detrimental porosity (gas pores, lack-of-fusion defects) is critical for the mechanical integrity, particularly fatigue life, of load-bearing implants. Conversely, for porous structures designed for osseointegration, controlling the level and type of porosity is essential.
- Mitigation:
- Powder Quality: Using high-quality, spherical powder with controlled particle size distribution and low gas content (Met3dp’s focus on advanced powder production is key here).
- Parameter Optimization: Developing and validating optimal process parameters (laser/beam power, scan speed, layer thickness, hatch spacing) for the specific material and machine.
- Process Atmosphere: Maintaining a clean, controlled inert gas atmosphere (L-PBF) or vacuum (SEBM) to prevent oxidation and contamination.
- Post-Processing: Implementing Hot Isostatic Pressing (HIP) is the most effective way to close internal microporosity and ensure maximum density for critical applications. Porosity control AM implants is a combination of prevention during printing and correction via HIP.
3. Support Structure Removal:
- Issue: Removing support structures, especially complex internal supports within lattice structures or channels, can be difficult, time-consuming, and costly. Incomplete removal can compromise biocompatibility or functionality, while aggressive removal can damage the implant.
- Mitigation:
- DfAM: Designing for minimal support usage and easy access (self-supporting angles, optimized orientation, breakaway supports).
- Optimized Support Generation: Using advanced software to create easily removable support types.
- Skilled Labor: Employing trained technicians with expertise in support removal techniques.
- Advanced Techniques: Exploring methods like electrochemical machining where applicable, although often complex to implement.
4. Powder Management (Handling, Quality, Recycling):
- Issue: Metal powders (especially reactive ones like Titanium) require careful handling to avoid contamination (e.g., cross-material, oxygen pickup) and ensure operator safety (inhalation/explosion risk). Maintaining powder quality throughout its lifecycle, including validation of recycled powder properties, is crucial for consistent part quality.
- Mitigation:
- Controlled Environments: Using dedicated powder handling stations, inert atmosphere glove boxes.
- Strict Protocols: Implementing rigorous procedures for powder loading, unloading, sieving, storage, and traceability.
- Powder Characterization: Regularly testing virgin and recycled powder lots (chemistry, PSD, morphology, flowability).
- Recycling Strategy: Developing validated protocols for powder reuse, defining limits on the number of reuse cycles based on material testing.
5. Process Validation and Consistency:
- Issue: Ensuring that the entire AM process, from powder to finished part, is repeatable and consistently produces implants meeting specifications is a major undertaking, especially given the complexity of the technology and the criticality of the application.
- Mitigation:
- Robust Quality Management System (QMS): Implementing and adhering to ISO 13485 standards for medical device manufacturing.
- Machine Qualification (IQ/OQ/PQ): Thoroughly validating machine installation, operation, and performance.
- Process Parameter Validation: Locking down optimized and validated process parameters.
- In-Process Monitoring: Utilizing sensors and monitoring tools (e.g., melt pool monitoring, thermal imaging) where available to track build consistency.
- Rigorous Testing: Comprehensive testing of final parts (dimensional, mechanical, NDT). The entire implant validation process must be documented.
6. Regulatory Hurdles:
- Issue: Navigating the regulatory landscape (e.g., FDA 510(k) clearance or Premarket Approval (PMA) in the US, CE marking under MDR in Europe) for additively manufactured medical devices is complex and evolving. Regulators require extensive evidence of safety and efficacy, including detailed process validation, material characterization, mechanical testing, biocompatibility testing, and cleaning validation.
- Mitigation:
- Expertise: Partnering with manufacturers and consultants experienced in regulatory submissions for AM devices.
- Standards Compliance: Adhering to relevant ASTM and ISO standards for AM processes and materials.
- Thorough Documentation: Maintaining meticulous records of the entire design, manufacturing, and testing process. Engaging with regulatory bodies early in the development process.
7. Cost and Scalability:
- Issue: While cost-effective for custom or complex parts, the cost per part for metal AM can still be higher than traditional mass production methods for simpler, standard implants. Scaling production to very high volumes can also present challenges related to machine throughput, powder supply, and post-processing capacity.
- Mitigation:
- DfAM for Cost: Optimizing designs to reduce build time and support material.
- Process Efficiency: Nesting multiple parts per build, optimizing machine utilization.
- Automation: Implementing automation in post-processing steps where feasible.
- Strategic Application: Focusing AM on applications where its benefits (customization, complexity, performance) provide the most value over traditional methods.
Addressing these metal AM challenges medical requires significant investment in technology, expertise, and process control. Companies like Met3dp, with their focus on both advanced metal 3D printing equipment and high-quality powder production, are well-positioned to help medical device manufacturers overcome these hurdles and successfully leverage AM for innovative orthopedic solutions.
Selecting the Right Metal 3D Printing Partner for Medical Device Manufacturing
Choosing a manufacturing partner for critical medical devices like orthopedic implants is a decision with significant implications for product quality, patient safety, regulatory compliance, and time-to-market. While numerous companies offer metal additive manufacturing services, not all possess the specific expertise, rigorous quality systems, and validated processes required for the demanding medical field. For engineers and procurement managers, conducting thorough due diligence is essential. Evaluating AM service bureaus requires looking beyond basic printing capabilities.
Here are key criteria for selecting the right medical device AM supplier selection:
1. Quality Management System & Certifications:
- ISO 13485: This is the international standard for Quality Management Systems for Medical Devices. Certification to ISO 13485 is non-negotiable for any manufacturer producing implants. It demonstrates a commitment to rigorous process control, risk management, traceability, documentation, and regulatory compliance specific to the medical industry. Ask for their certificate and scope. ISO 13485 certified 3D printing is a baseline requirement.
- ISO 9001: Certification for general quality management provides a foundation but is insufficient alone for medical device manufacturing.
- Facility Audits: Plan to conduct your own quality audit of the potential supplier’s facility to verify their QMS implementation, cleanliness, process controls, and documentation practices firsthand.
2. Medical Device & Orthopedic Expertise:
- Proven Track Record: Look for demonstrated experience specifically in manufacturing orthopedic implants or components using metal AM. Ask for case studies, examples of similar parts produced, and potentially anonymized client references.
- Materials Expertise: Deep understanding of medical-grade alloys like Ti-6Al-4V ELI and CoCrMo, including their metallurgy, behavior during AM processing, and appropriate post-processing requirements.
- Regulatory Knowledge: Familiarity with FDA regulations (e.g., 510(k), PMA), EU Medical Device Regulation (MDR), and relevant ASTM/ISO standards for materials and processes.
- Engineering Support: Ability to provide Design for Additive Manufacturing (DfAM) guidance, collaborating with your team to optimize implant designs for printability, performance, and cost-effectiveness.
3. Technological Capabilities & Capacity:
- Appropriate AM Technology: Do they operate the right type of machines (e.g., SEBM, L-PBF) for your specific material and application needs? Understand the differences in precision, surface finish, and material properties between technologies (as discussed in Part 2). Met3dp, for instance, specializes in advanced SEBM printers, ideal for high-quality titanium implants.
- Machine Park: Sufficient number of well-maintained machines to handle your required volume and provide redundancy, minimizing lead time risks due to machine downtime.
- Comprehensive In-House Post-Processing: Ideally, the partner should have extensive in-house capabilities for critical post-processing steps: stress relief, HIP (or a validated partnership), precision CNC machining, various surface finishing methods, validated cleaning lines, and passivation. Relying heavily on multiple external subcontractors increases complexity and risk.
- Powder Management: Robust systems for powder sourcing, testing, handling, storage, and recycling, ensuring traceability and consistent quality. Companies like Met3dp, which manufacture their own high-quality metal powders using advanced atomization techniques, offer significant advantages in material control and expertise.
4. Robust Quality Control & Inspection:
- Material Traceability: Lot traceability for all raw materials (powder) throughout the manufacturing process.
- Process Monitoring & Validation: Documented evidence of process validation (IQ/OQ/PQ) for AM machines and critical post-processing steps. Use of in-process monitoring where applicable.
- Inspection Equipment: Calibrated inspection tools, including Coordinate Measuring Machines (CMM) for dimensional verification, surface profilometers, and potentially Non-Destructive Testing (NDT) capabilities like CT scanning for internal integrity checks.
5. Communication, Collaboration & Transparency:
- Partnership Approach: Look for a supplier who acts as a collaborative partner, willing to share expertise, provide regular updates, and work through challenges together.
- Responsiveness: Clear communication channels and timely responses to inquiries and requests.
- Transparency: Willingness to share relevant process data, validation summaries, and quality documentation (within the bounds of confidentiality agreements).
Evaluation Checklist Summary:
| Criterion | Key Considerations | Importance |
|---|---|---|
| ISO 13485 Certification | Mandatory for medical devices; verify scope. | Critical |
| Orthopedic AM Experience | Proven track record with similar implants; case studies. | High |
| Materials Expertise | Deep knowledge of Ti-6Al-4V ELI, CoCrMo; understanding of AM effects. | High |
| DfAM Support | Ability to assist in optimizing designs for AM. | High |
| AM Technology Fit | Suitable machines (SEBM/L-PBF) for the application; machine condition & calibration. | High |
| In-House Post-Processing | Comprehensive capabilities (Heat Treat, HIP, Machining, Cleaning, Finishing). | High |
| Powder Management | Strict controls for sourcing, handling, testing, recycling; traceability. | High |
| QMS Implementation | Rigorous process validation, inspection capabilities (CMM, NDT), documentation. | Critical |
| Communication | Collaborative approach, responsiveness, transparency. | Medium |
| Cost & Lead Time | Competitive pricing, reliable delivery timelines. | Medium |
Export to Sheets
Selecting the right orthopedic implant manufacturing partner is a critical step. Companies like Met3dp, with decades of collective expertise in metal additive manufacturing, advanced SEBM printers, in-house powder production capabilities, and a commitment to quality, represent the type of comprehensive partner needed for success in this field. Learning more About Us and our commitment to enabling next-generation manufacturing can provide valuable insight into selecting a qualified partner.
Understanding Cost Drivers and Lead Times for Patient-Specific Implants
While metal additive manufacturing enables the creation of highly customized and complex orthopedic implants, understanding the factors that influence production cost and delivery timelines is essential for project planning, budgeting, and managing expectations. Unlike traditional mass production where costs per part decrease significantly with volume, the cost of 3D printed orthopedic implants, especially patient-specific ones, has a different structure.
Key Cost Drivers:
- Material Cost:
- Powder Type: Medical-grade powders like Ti-6Al-4V ELI and CoCrMo are inherently expensive raw materials. Cost varies between alloy types.
- Part Volume & Supports: The actual amount of powder consumed to build the part and its necessary support structures directly impacts cost. Larger, denser parts use more material. Efficient DfAM aims to minimize volume and support material.
- Powder Recycling: The efficiency and validation of the supplier’s powder recycling process influence the effective material cost. Inefficient recycling increases costs.
- AM Machine Time:
- Build Duration: Calculated based on the part’s height (number of layers), the volume of material being fused per layer, and the specific machine’s deposition rate and scanning strategy. Taller parts or multiple parts nested inefficiently take longer.
- Machine Overhead: Includes depreciation of expensive AM equipment, energy consumption (especially for high-temperature processes like SEBM), maintenance, and operational costs, factored into an hourly machine rate.
- Labor Costs:
- Pre-Processing: CAD file preparation, build layout planning (nesting), slicing, and parameter assignment.
- Machine Setup & Operation: Loading powder, setting up the build, monitoring the process, and unloading the finished build.
- Post-Processing Labor: This can be a significant cost component. It includes manual or semi-automated part removal from the build plate, intricate support removal (often requires skilled technicians), setup and operation of CNC machines, manual finishing/polishing, cleaning, inspection, and packaging.
- Post-Processing Steps:
- Specific Treatments: Costs associated with required steps like thermal stress relief, Hot Isostatic Pressing (HIP – often outsourced, adding cost and lead time), CNC machining (machine time + labor + tooling), surface treatments (blasting, polishing, coating), and validated cleaning/passivation processes. Each step adds cost.
- Quality Assurance & Validation:
- Inspection: Time and resources for dimensional inspection (CMM), surface analysis, NDT (if required), and documentation review.
- Process Validation: Amortized costs of initial process validation (IQ/OQ/PQ) and ongoing process monitoring and control efforts required under ISO 13485.
- Development & Engineering (NRE):
- For novel or highly complex patient-specific implants, there may be non-recurring engineering (NRE) costs associated with extensive DfAM, custom process development, specific tooling or fixtures, and potentially unique validation testing.
- Order Volume:
- While not as impactful as in traditional manufacturing, some economies of scale exist. Printing multiple parts in a single build (efficient nesting) utilizes machine time better. Batch processing during post-processing steps (like heat treatment, HIP, cleaning) can also reduce per-part costs compared to single-piece flow.
Typical Lead Time Factors:
The lead time custom medical devices produced via AM is influenced by several stages:
- Design & Approval (Variable): Time taken by the clinical/engineering team to finalize the implant design based on patient scans and surgical planning. Requires close collaboration.
- Quoting & Order Processing (1-5 days): Supplier review of the design, DfAM checks, quote generation, and order confirmation.
- Build Planning & Queue Time (1-10 days): Scheduling the build on an available machine, optimizing the build layout. Can vary significantly based on supplier workload.
- Printing / Build Time (1-4 days): Actual time the part spends printing in the AM machine. Highly dependent on part size/complexity.
- Post-Processing (1-4 weeks): This is often the longest portion of the lead time. It includes:
- Cool-down and stress relief (hours to 1 day)
- Removal from plate and support removal (hours to days, depending on complexity)
- Shipping to/from HIP facility and HIP cycle time (typically 3-7 days total)
- CNC Machining (setup and runtime, potentially several days for complex parts)
- Finishing, cleaning, passivation (1-3 days)
- Final inspection (1-2 days)
- Shipping (1-5 days): Transit time to the customer.
Overall Estimated Lead Time: For a moderately complex patient-specific metal orthopedic implant, a typical total lead time from finalized design to shipment might range from 3 to 8 weeks. This is highly variable based on all the factors above. Urgent procedures might sometimes be expedited, but usually at a significant cost premium and potentially bypassing some non-critical steps (requiring careful risk assessment).
It is crucial for procurement managers and engineers to engage with potential AM partners early, provide complete design information, and discuss specific requirements to get accurate quotes for both cost (metal AM pricing factors) and lead time.
Frequently Asked Questions (FAQ) about Metal 3D Printed Orthopedic Implants
As metal additive manufacturing becomes more prevalent in orthopedics, engineers, surgeons, and procurement professionals often have questions about the technology and its application. Here are answers to some frequently asked questions (3D printed implant FAQ):
1. Are 3D printed metal implants FDA cleared or CE marked?
- It’s important to understand that the regulatory bodies (like the FDA in the US or Notified Bodies for CE marking under MDR in Europe) clear or approve the final medical device and the validated manufacturing process used to produce it, not the additive manufacturing technology itself in isolation. An implant manufacturer must submit detailed documentation demonstrating the safety and efficacy of their specific implant design, manufactured using a specific, validated AM process (including powder specifications, machine parameters, post-processing steps) under a certified Quality Management System (like ISO 13485). Many AM-produced orthopedic implants have received FDA clearance (often via the 510(k) pathway citing substantial equivalence to traditionally manufactured devices) and CE marking, but each device requires its own submission and approval based on its specific design and manufacturing controls.
2. How does the fatigue life and mechanical strength of AM implants compare to traditionally manufactured (e.g., wrought or cast) implants?
- When produced using optimized parameters and subjected to appropriate post-processing, particularly Hot Isostatic Pressing (HIP), metal AM implants made from materials like Ti-6Al-4V ELI and CoCrMo can exhibit mechanical properties (yield strength, ultimate tensile strength, fatigue strength) that meet or even exceed the requirements specified in ASTM or ISO standards for wrought or cast materials used in surgical implants. HIP is critical for minimizing internal porosity, which significantly benefits fatigue performance. Numerous studies have demonstrated the excellent fatigue life of properly processed AM orthopedic components, making them suitable for long-term load-bearing applications.
3. Can the complex internal lattice structures in 3D printed implants be effectively cleaned and sterilized?
- Yes, but it requires careful consideration during the design phase (DfAM) and validated processes. Designs must ensure that porous structures are fully interconnected (no closed pores) to allow cleaning solutions and sterilizing agents (like steam in an autoclave) to penetrate effectively. The manufacturing process must include rigorous, validated cleaning protocols specifically designed to remove residual powder particles from these intricate structures. Techniques often involve ultrasonic cleaning, high-pressure rinsing, and multiple cycles with specific detergents. Validation typically involves testing for residual contaminants and ensuring the sterilization process achieves the required sterility assurance level (SAL).
4. What information does a metal AM service provider like Met3dp need to provide an accurate quote for a custom orthopedic implant?
- To provide an accurate quote and assess manufacturability, suppliers typically need:
- 3D CAD Model: A precise digital model of the implant, preferably in a standard format like STEP (.stp/.step).
- Material Specification: Clearly defined alloy (e.g., Ti-6Al-4V ELI) and reference to the relevant standard (e.g., ASTM F136 chemistry and mechanical properties required).
- Technical Drawings (Optional but helpful): 2D drawings indicating critical dimensions, tolerances, surface finish requirements (Ra values for specific surfaces), and any specific features or annotations.
- Post-Processing Requirements: Specify mandatory steps like HIP, required machining operations (highlighting critical surfaces), desired surface finishes, and any special cleaning or marking needs.
- Quantity: Number of implants required (even if only one for patient-specific cases).
- Required Delivery Date: Desired timeline to assess feasibility.
- Applicable Standards: Any other specific industry or regulatory standards the implant must meet.
5. Is metal additive manufacturing suitable for large-volume production of orthopedic implants?
- Metal AM technology is continually improving in speed and efficiency, making it increasingly viable for larger production runs. However, for very high volumes (tens or hundreds of thousands per year) of simple, standardized implants, traditional manufacturing methods like casting or forging followed by machining often remain more cost-effective due to established infrastructure and economies of scale. Metal AM’s primary strength currently lies in:
- Patient-specific implants (volume of one).
- Complex designs incorporating features like integrated porous structures or topology optimization, which are difficult or impossible to make traditionally.
- Low-to-medium volume production runs.
- Bridge manufacturing or market entry before investing in expensive traditional tooling. As AM technologies mature further, particularly regarding print speed, automation, and powder cost reduction, the threshold for cost-effective serial production using AM is expected to rise, making it competitive for a broader range of orthopedic implants, even at higher volumes.
These metal AM orthopedics questions highlight common areas of interest. Partnering with knowledgeable suppliers who can provide clear answers and guidance is essential for successful implementation.
Conclusion: Embracing Additive Manufacturing for the Future of Orthopedic Care
Metal additive manufacturing is no longer a futuristic novelty in orthopedics; it is a powerful, validated, and increasingly indispensable tool shaping the present and future of orthopedic implants. Its ability to create patient-specific devices with intricate geometries and integrated features like biomimetic porous structures offers unprecedented advantages in tackling complex orthopedic challenges and advancing personalized medicine. From enhancing osseointegration in joint replacements and spinal cages to enabling precise reconstructions in CMF and trauma surgery, the metal AM impact medical field is profound and continues to grow.
We’ve explored the journey of a metal AM orthopedic implant – from understanding its diverse applications and the unique benefits AM offers over traditional methods, to selecting the right biocompatible materials like Ti-6Al-4V ELI and CoCrMo. We delved into the critical importance of Design for Additive Manufacturing (DfAM), the achievable levels of precision, the essential nature of comprehensive post-processing steps like HIP and machining, and the common challenges that require expertise and rigorous process control to overcome.
Successfully leveraging this technology demands careful consideration of partner selection, focusing on suppliers with proven medical device expertise, robust ISO 13485 certified quality systems, and the right technological capabilities. Understanding the cost drivers and typical lead times associated with custom AM implants allows for better project planning and realistic expectations.
The journey towards truly personalized orthopedic care is accelerating, and metal additive manufacturing is firmly in the driver’s seat for complex implants. As the technology continues to evolve – with new materials, faster machines, enhanced software tools leveraging AI for design, and improved process monitoring – its capabilities and cost-effectiveness will only increase.
For medical device companies, surgeons, and engineers looking to innovate and provide the best possible solutions for patients, embracing metal AM is becoming essential. Partnering with a knowledgeable and capable additive manufacturing partnership medical provider is key to navigating this technological landscape successfully.
Met3dp stands at the forefront of this revolution, offering comprehensive solutions that span industry-leading SEBM metal 3D printing equipment, advanced high-quality metal powders manufactured in-house, and deep application development expertise. We partner with organizations across aerospace, medical, automotive, and industrial sectors to implement 3D printing and accelerate their digital manufacturing transformations. As a leader in metal AM equipment and materials, Met3dp delivers cutting-edge systems and powders to enable next-generation manufacturing.
Ready to explore how metal additive manufacturing can power your organization’s orthopedic device goals? Contact the experts at Met3dp today or visit our website at https://met3dp.com/ to learn more about our capabilities and how we can help you revolutionize patient care through advanced manufacturing.
Share On
MET3DP Technology Co., LTD is a leading provider of additive manufacturing solutions headquartered in Qingdao, China. Our company specializes in 3D printing equipment and high-performance metal powders for industrial applications.
Inquiry to get best price and customized Solution for your business!
Related Articles
About Met3DP
Recent Update
Our Product
CONTACT US
Any questions? Send us message now! We’ll serve your request with a whole team after receiving your message.

Metal Powders for 3D Printing and Additive Manufacturing
COMPANY
PRODUCT
cONTACT INFO
- Qingdao City, Shandong, China
- [email protected]
- [email protected]
- +86 19116340731