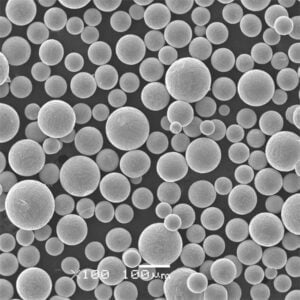
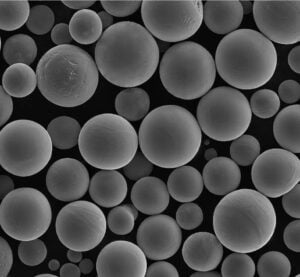
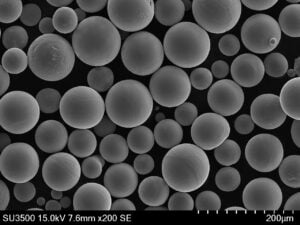
ASTM F136: The Ultimate Guide
Table of Contents
Welcome, dear reader! Today, we’re diving deep into the fascinating world of ASTM F136. Now, you might be wondering, “What in the world is ASTM F136?” Don’t worry, I’ve got you covered. By the end of this extensive guide, you’ll know everything there is to know about this remarkable material.
Overview of ASTM F136
ASTM F136, also known as Titanium 6Al-4V ELI (Extra Low Interstitial), is a titanium alloy widely recognized for its use in the medical field. This alloy stands out due to its biocompatibility, corrosion resistance, and excellent mechanical properties, making it a go-to material for medical implants, surgical instruments, and other critical applications.
Key Details of ASTM F136
- Composition: Titanium, Aluminum, Vanadium
- Properties: High strength, low weight, corrosion resistance, biocompatibility
- Applications: Medical implants, surgical instruments, aerospace components
- Specifications: ASTM F136 standard defines the quality and characteristics of the alloy
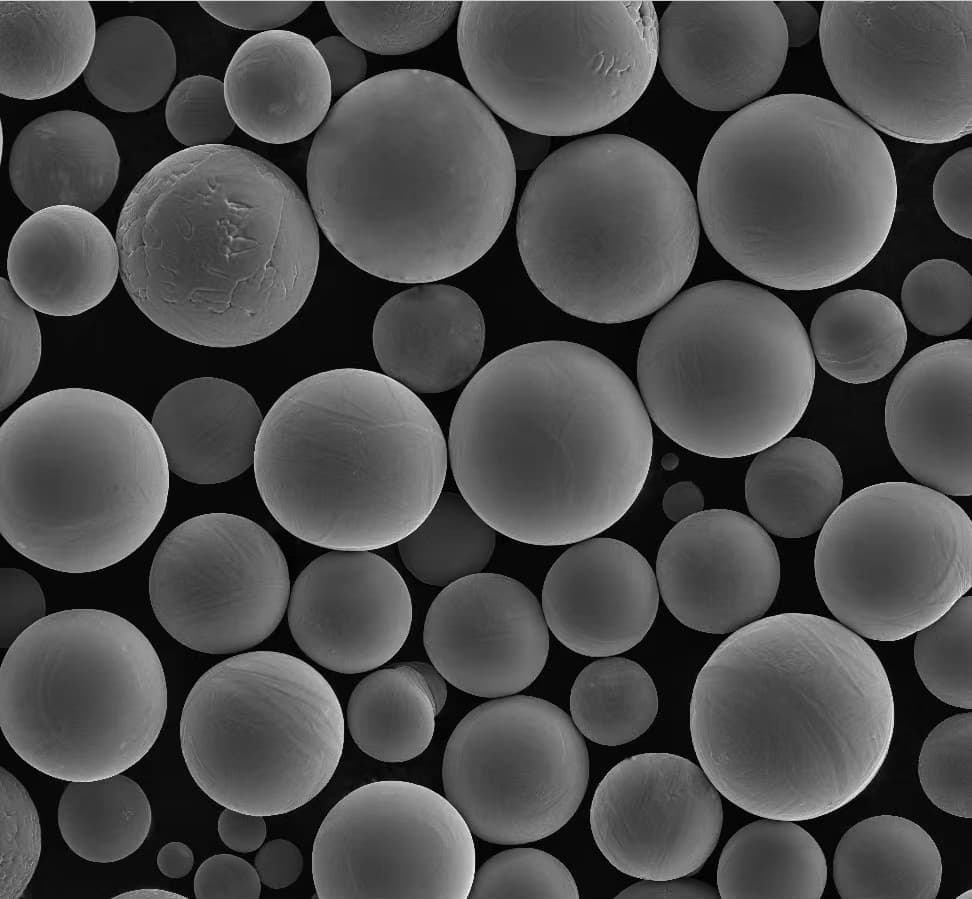
Types, Composition, Properties, and Characteristics
To truly appreciate ASTM F136, it’s essential to understand its composition, properties, and characteristics. Let’s break it down:
Composition of ASTM F136
| Element | Percentage |
|---|---|
| Titanium (Ti) | 88 – 90% |
| Aluminum (Al) | 5.5 – 6.75% |
| Vanadium (V) | 3.5 – 4.5% |
| Oxygen (O) | ≤ 0.13% |
| Carbon (C) | ≤ 0.08% |
| Nitrogen (N) | ≤ 0.05% |
| Hydrogen (H) | ≤ 0.0125% |
| Iron (Fe) | ≤ 0.25% |
Properties and Characteristics of ASTM F136
| Property | Description |
|---|---|
| Density | 4.43 g/cm³ |
| Young’s Modulus | 110 GPa |
| Ultimate Tensile Strength | 860 MPa |
| Yield Strength | 795 MPa |
| Elongation at Break | 15% |
| Hardness | 300 HV |
| Thermal Conductivity | 6.7 W/m·K |
| Electrical Resistivity | 1.7 µΩ·m |
| Biocompatibility | Excellent (meets ASTM F136 standards) |
| Corrosion Resistance | High (resistant to bodily fluids and chemicals) |
Applications of ASTM F136
ASTM F136 is primarily used in the medical field, but its applications extend to other industries due to its unique properties.
Medical Applications
| Application | Description |
|---|---|
| Orthopedic Implants | Hip replacements, knee joints, spinal implants |
| Dental Implants | Tooth roots, abutments, bridges |
| Surgical Instruments | Scalpels, forceps, retractors |
| Craniofacial Implants | Plates, screws for reconstructive surgery |
| Cardiovascular Implants | Heart valves, stents |
Aerospace Applications
| Application | Description |
|---|---|
| Aerospace Fasteners | Bolts, nuts, and screws for aircraft assembly |
| Structural Components | Airframe structures, landing gear |
| Engine Parts | Turbine blades, compressor disks |
Industrial Applications
| Application | Description |
|---|---|
| Chemical Processing | Equipment for chemical plants, reactors |
| Marine Applications | Shipbuilding, offshore drilling components |
| Sporting Goods | High-performance bicycles, golf clubs |
Specifications, Sizes, Grades, Standards
When working with ASTM F136, it’s crucial to adhere to the specified standards to ensure the material’s integrity and performance.
Specifications and Standards
| Standard | Description |
|---|---|
| ASTM F136 | Standard specification for wrought titanium-6Aluminum-4Vanadium ELI (extra low interstitial) alloy for surgical implant applications. |
Sizes and Grades
| Size Range | Description |
|---|---|
| Bars | Diameter: 6mm to 150mm |
| Sheets | Thickness: 0.5mm to 5mm |
| Plates | Thickness: 5mm to 100mm |
| Wires | Diameter: 0.1mm to 10mm |
| Grades | Grade 23 (Ti 6Al-4V ELI) |
Suppliers and Pricing Details
Finding the right supplier is key to ensuring you get high-quality ASTM F136 material. Here are some reputable suppliers and their pricing details.
Top Suppliers
| Supplier | Description | Pricing (Approximate) |
|---|---|---|
| ATI Metals | Leading global manufacturer and supplier of titanium and other specialty materials. | $50 – $100 per kg |
| Timet (Titanium Metals Corporation) | Major producer of titanium-based products with a focus on aerospace and medical applications. | $60 – $110 per kg |
| VSMPO-AVISMA | Largest titanium producer globally, supplying high-quality titanium alloys. | $55 – $105 per kg |
| Toho Titanium | Japanese supplier known for high purity and advanced titanium products. | $65 – $115 per kg |
| Arcam AB (GE Additive) | Specializes in additive manufacturing and advanced materials for medical and aerospace sectors. | $70 – $120 per kg |
Comparing Pros and Cons
When considering ASTM F136 for your application, it’s essential to weigh its advantages and limitations.
Advantages of ASTM F136
| Advantage | Description |
|---|---|
| Biocompatibility | Excellent compatibility with human tissues, making it ideal for medical implants. |
| Corrosion Resistance | Highly resistant to bodily fluids and chemicals, ensuring longevity. |
| Mechanical Properties | High strength-to-weight ratio, making it strong yet lightweight. |
| Versatility | Suitable for a wide range of applications, from medical to aerospace. |
Limitations of ASTM F136
| Limitation | Description |
|---|---|
| Cost | Relatively expensive compared to other materials. |
| Machinability | Requires specialized equipment and techniques for machining. |
| Availability | May have longer lead times due to high demand and specific manufacturing processes. |
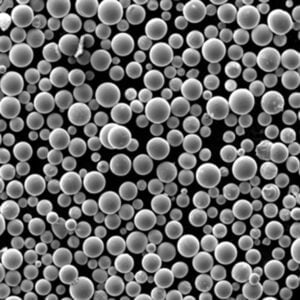
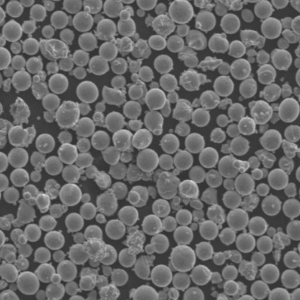
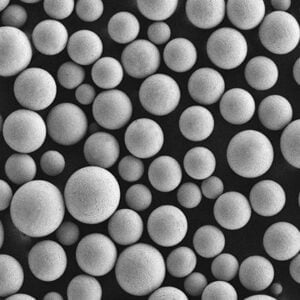
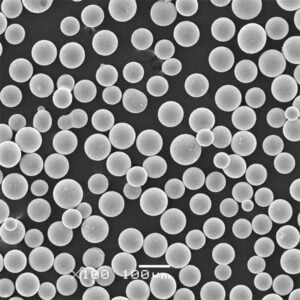
Specific Metal Powder Models
In the realm of additive manufacturing and advanced applications, several specific metal powder models of ASTM F136 stand out. Here are some notable examples:
Top Metal Powder Models
| Model | Description |
|---|---|
| Ti64 ELI by Arcam AB | High-purity titanium powder for Electron Beam Melting (EBM) technology. |
| TLS Ti6Al4V ELI by TLS Technik | High-quality powder for selective laser melting (SLM) and other additive manufacturing processes. |
| AP&C Ti-6Al-4V ELI by GE Additive | Spherical titanium powder designed for optimal flowability and packing density in additive manufacturing. |
| Ti-6Al-4V ELI by Carpenter Additive | High-performance powder for various 3D printing technologies, ensuring consistent quality and properties. |
| AMTi-6Al-4V ELI by Tekna | Plasma atomized titanium powder for superior performance in additive manufacturing. |
| Ti-6Al-4V ELI by Oerlikon Metco | High-quality powder for laser cladding, additive manufacturing, and other advanced processes. |
| Ti-6Al-4V ELI by LPW Technology | Engineered powder for high-strength, lightweight applications in aerospace and medical fields. |
| Ti-6Al-4V ELI by Praxair Surface Technologies | Consistent and high-purity powder for demanding additive manufacturing applications. |
| Ti-6Al-4V ELI by Sandvik | Premium titanium powder for additive manufacturing, ensuring excellent mechanical properties and biocompatibility. |
| Ti-6Al-4V ELI by Renishaw | Versatile powder for a wide range of additive manufacturing technologies, offering high performance and reliability. |
Comparative Analysis of Metal Powder Models
To help you make an informed decision, let’s compare these metal powder models based on various parameters.
Performance Comparison
| Model | Flowability | Packing Density | Purity Level | Price Range |
|---|---|---|---|---|
| Ti64 ELI by Arcam AB | Excellent | High | Ultra-High | $100 – $150/kg |
| TLS Ti6Al4V ELI by TLS Technik | Very Good | High | High | $90 – $140/kg |
| AP&C Ti-6Al-4V ELI by GE Additive | Excellent | Very High | Ultra-High | $110 – $160/kg |
| Ti-6Al-4V ELI by Carpenter Additive | Very Good | High | High | $95 – $145/kg |
| AMTi-6Al-4V ELI by Tekna | Excellent | High | Ultra-High | $105 – $155/kg |
| Ti-6Al-4V ELI by Oerlikon Metco | Very Good | High | High | $100 – $150/kg |
| Ti-6Al-4V ELI by LPW Technology | Excellent | Very High | Ultra-High | $110 – $160/kg |
| Ti-6Al-4V ELI by Praxair Surface Technologies | Very Good | High | High | $95 – $145/kg |
| Ti-6Al-4V ELI by Sandvik | Excellent | Very High | Ultra-High | $110 – $160/kg |
| Ti-6Al-4V ELI by Renishaw | Excellent | High | High | $100 – $150/kg |
Pros and Cons Comparison
| Model | Pros | Cons |
|---|---|---|
| Ti64 ELI by Arcam AB | High purity, excellent flowability, reliable performance | Higher cost compared to some alternatives |
| TLS Ti6Al4V ELI by TLS Technik | Consistent quality, good price-to-performance ratio | Slightly lower packing density compared to others |
| AP&C Ti-6Al-4V ELI by GE Additive | Ultra-high purity, very high packing density | Higher price range |
| Ti-6Al-4V ELI by Carpenter Additive | High performance, consistent properties | Mid to high price range |
| AMTi-6Al-4V ELI by Tekna | Superior performance, high purity, excellent for additive manufacturing | Higher cost |
| Ti-6Al-4V ELI by Oerlikon Metco | Reliable performance, good flowability | Mid to high price range |
| Ti-6Al-4V ELI by LPW Technology | Ultra-high purity, excellent packing density | Higher price range |
| Ti-6Al-4V ELI by Praxair Surface Technologies | Consistent quality, good performance | Mid to high price range |
| Ti-6Al-4V ELI by Sandvik | Premium quality, excellent mechanical properties | Higher cost |
| Ti-6Al-4V ELI by Renishaw | High reliability, versatile applications | Mid to high price range |
FAQ
What does ASTM F136 stand for?
ASTM F136 refers to the standard specification for wrought titanium-6Aluminum-4Vanadium ELI (extra low interstitial) alloy used primarily for surgical implant applications.
Why is ASTM F136 preferred for medical implants?
ASTM F136 is preferred for medical implants due to its excellent biocompatibility, corrosion resistance, and high mechanical strength. These properties ensure that the material can withstand the harsh environment of the human body and remain functional over long periods.
What are the primary elements in ASTM F136?
The primary elements in ASTM F136 are Titanium (Ti), Aluminum (Al), and Vanadium (V), with Titanium being the predominant component.
How is ASTM F136 typically manufactured?
ASTM F136 is manufactured through various processes including forging, rolling, and heat treatment to achieve the desired mechanical properties and ensure compliance with the standard specifications.
Apart from medical, where else is ASTM F136 used?
Beyond the medical field, ASTM F136 is also used in aerospace for structural components, fasteners, and engine parts, as well as in industrial applications like chemical processing and marine engineering.
Is ASTM F136 suitable for 3D printing?
Yes, ASTM F136 is widely used in additive manufacturing, especially in the form of titanium powder for 3D printing technologies like Electron Beam Melting (EBM) and Selective Laser Melting (SLM).
Is ASTM F136 expensive?
ASTM F136 is relatively more expensive than other materials due to its high performance and specialized applications, with prices typically ranging from $50 to $160 per kilogram depending on the supplier and form (bars, sheets, powder, etc.).
Conclusion
There you have it – a comprehensive guide to ASTM F136! We’ve covered everything from its composition and properties to its applications, specifications, and even a deep dive into various metal powder models for advanced manufacturing. Whether you’re considering ASTM F136 for medical implants, aerospace components, or any other high-performance application, this guide should serve as your go-to resource.
know more 3D printing processes
Additional FAQs on ASTM F136
1) What differentiates ASTM F136 (Grade 23) from ASTM F1472 (Grade 5) Ti-6Al-4V?
- ASTM F136 is the ELI (Extra-Low Interstitial) version with tighter limits on O, N, C, H, improving fracture toughness and fatigue performance for implants. F1472 allows higher interstitials and is typically used for non-implant applications.
2) Which tests are mandatory to certify material to ASTM F136?
- Chemical analysis (including interstitials), tensile properties, reduction of area/elongation, microstructural verification (alpha/beta), and melt practice traceability. For implants, many OEM specs also require low inclusion content and fracture toughness or fatigue testing.
3) How does surface condition affect implant performance for ASTM F136?
- Surface roughness, residual stress, and contamination strongly influence fatigue strength and osseointegration. Polishing, blasting, acid etch, or TiO2 anodizing are used per device function; all must preserve ELI cleanliness.
4) Is recycled titanium allowed in ASTM F136 melts?
- The standard permits revert with strict control; however, many medical OEMs cap revert content and require documented segregation and inclusion control to meet risk-management and regulatory expectations.
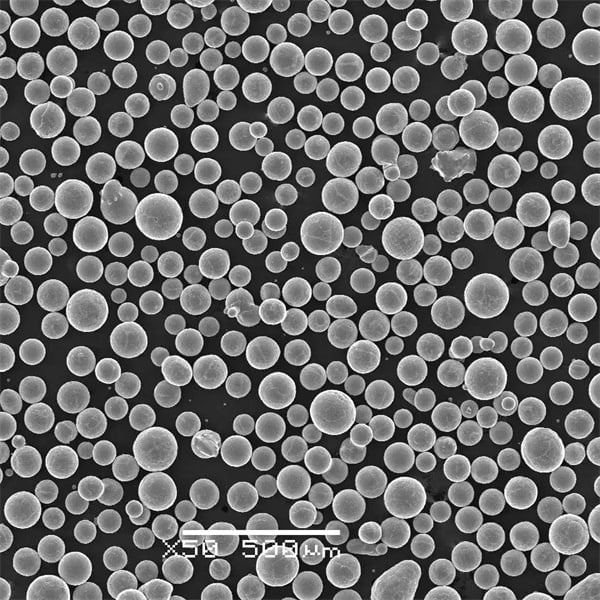
5) What additive manufacturing (AM) considerations apply to ASTM F136 powder?
- AM-grade powder requires high sphericity, tight PSD (e.g., 15–45 μm for LPBF), and low O/N/H. Post-build HIP + stress relief is common to improve fatigue. Powder reuse must be controlled to limit oxygen pickup and PSD drift per ISO/ASTM 52907.
2025 Industry Trends for ASTM F136
- AM dominance in ortho/dental: Growing share of acetabular cups, cages, and patient-specific implants produced via LPBF/EBM using ASTM F136 powders, with routine HIP for fatigue-critical parts.
- Powder passports: End-to-end genealogy linking melt heat, PSD, O/N/H, reuse cycles, and build parameters becomes standard in MDR/FDA submissions.
- Low-helium strategies: Plasma/GA atomizers reduce helium reliance, cutting powder cost volatility while maintaining powder sphericity and cleanliness.
- Surface engineering: Controlled roughness and porous lattices for enhanced osseointegration, validated with standardized fatigue-on-porous coupons.
- Sustainability: Environmental Product Declarations (EPDs) and Scope 3 reporting increasingly required in tenders for implant supply chains.
2025 Snapshot: ASTM F136 Production and AM Benchmarks (indicative)
| Metric | 2023 | 2024 | 2025 YTD | Notes/Sources |
|---|---|---|---|---|
| Typical oxygen content in bar (% wt) | 0.10–0.13 | 0.09–0.12 | 0.08–0.11 | Within ASTM F136 limit ≤0.13 |
| LPBF density (as-built, %) | 99.5–99.8 | 99.6–99.9 | 99.7–99.95 | Process optimized; preheat strategies |
| HIPed fatigue improvement (R=0.1, 10^7 cycles) | +20–40% | +25–45% | +25–50% | Depends on surface and lattice |
| Powder reuse cycles (with O control) | 6–10 | 8–12 | 10–15 | With top-up and sieving management |
| Pump-down time EBM (min) | 45–90 | 40–80 | 35–70 | Cryopump adoption |
References: ASTM F136; ISO 5832-3; ISO/ASTM 52907/52908; FDA, EU MDR guidance; OEM and supplier notes (GE Additive/AP&C, Carpenter Additive, Höganäs); NIST AM Bench.
Latest Research Cases
Case Study 1: Improving Fatigue of LPBF ASTM F136 Acetabular Cups via HIP and Surface Control (2025)
- Background: An orthopedic manufacturer observed scatter in rotating-bending fatigue on porous-backed cups.
- Solution: Implemented powder passport tracking (O/N/H, PSD, reuse count), HIP at 920°C/100 MPa/2 h, and controlled grit blast followed by acid etch to target Ra 1.2–1.8 μm on functional surfaces.
- Results: Endurance limit +32% at 10^7 cycles; between-lot COV reduced from 18% to 9%; CT-indicated pore clusters >150 μm reduced by 70%.
Case Study 2: Machined vs AM ASTM F136 Spinal Cages—Qualification Pathway (2024)
- Background: A spine device firm evaluated switching from machined bar to LPBF latticed cages to enhance fusion.
- Solution: Comparative qualification including chemistry, tensile, LCF/HCF fatigue, corrosion (ASTM F2129), and particulate shedding; validated with animal model histology for bone ingrowth.
- Results: AM design achieved equivalent static strength, 28% higher compressive fatigue limit, and 2× bone ingrowth area at 12 weeks; regulatory submission included full AM process validation and powder control plan.
Expert Opinions
- Prof. Todd Palmer, Professor of Engineering, Penn State
- Viewpoint: “For ASTM F136 in AM, oxygen control across powder lifecycle is the single most leverageable variable for fatigue—more than minor parameter tweaks.”
- Annika Ölme, VP Technology, GE Additive (Arcam EBM)
- Viewpoint: “Combining EBM preheat with HIP delivers consistent fatigue for porous implant structures while preserving osseointegration-friendly surfaces.”
- Dr. John Slotwinski, Director of Materials Engineering, Relativity Space
- Viewpoint: “Digital material passports linking melt, powder, and build data are becoming essential quality artifacts—healthcare regulators increasingly expect them.”
Practical Tools and Resources
- Standards
- ASTM F136 (Ti-6Al-4V ELI), ISO 5832-3 (surgical implants): https://www.astm.org | https://www.iso.org
- ISO/ASTM 52907 (AM feedstock), 52908 (AM post-processing), 52920 (qualification)
- Regulatory and guidance
- FDA guidance on AM of medical devices; EU MDR implantable device requirements
- Metrology and QA
- LECO (O/N/H), PSD: ASTM B822; density/flow: ASTM B212/B213; CT per ASTM E07
- AM process tools
- Simulation and build prep: Materialise Magics, Ansys Additive, Siemens NX AM
- NIST AM Bench datasets for Ti-6Al-4V process–structure–property correlations
- Surface and corrosion
- ASTM F2129 (corrosion of metallic implants), ISO 10993 (biocompatibility evaluation)
Last updated: 2025-10-16
Changelog: Added 5 focused FAQs; introduced a 2025 benchmark table for ASTM F136 production and AM use; provided two case studies (LPBF cups with HIP; spinal cage qualification); included expert viewpoints; compiled standards, regulatory, QA, and AM tools resources
Next review date & triggers: 2026-03-31 or earlier if ASTM/ISO implant standards update, regulators issue new AM guidance, or major OEMs revise powder passport and HIP best practices
Share On
MET3DP Technology Co., LTD is a leading provider of additive manufacturing solutions headquartered in Qingdao, China. Our company specializes in 3D printing equipment and high-performance metal powders for industrial applications.
Inquiry to get best price and customized Solution for your business!
Related Articles
Metal 3D Printing for U.S. Automotive Lightweight Structural Brackets and Suspension Components
Read More »About Met3DP
Recent Update
Our Product
CONTACT US
Any questions? Send us message now! We’ll serve your request with a whole team after receiving your message.